Take-home points
|
 |
Bios Dr. Berni was a retina research fellow at Bascom Palmer Eye Institute in Miami and is completing an ophthalmology residency at San Raffaele Hospital in Milan, Italy. Dr. Rosenfeld is a professor of ophthalmology at Bascom Palmer Eye Institute. DISCLOSURES: Research was supported by grants from Carl Zeiss Meditec, an unrestricted grant from the Research to Prevent Blindness, (New York, NY) and the National Eye Institute (P30EY014801, R01 EY028753). The funding organizations had no role in the design or conduct of the present research. Dr. Rosenfeld received research support from Carl Zeiss Meditec and research funding from Novartis. He’s also a consultant for AbbVie, Apellis, Bayer Pharmaceuticals, Boehringer-Ingelheim, Carl Zeiss Meditec, Character Biosciences, Genentech/Roche, InflammX Therapeutics, Ocudyne, Regeneron Pharmaceuticals, Sanofi and Unity Biotechnology. He also has equity interest in Apellis, Character Biosciences, InflammX, Ocudyne and Valitor. Dr. Berni has no financial disclosures. |
Predicting the progression of an eye with intermediate age-related macular degeneration to geographic atrophy is challenging, due to such factors as limitations of imaging biomarkers, variability in AMD’s phenotype and an incomplete understanding of the individual risk factors. Here, we’ll show how new imaging approaches can help more accurately stratify the risk of progression to GA.
The challenge of AMD
The threat of AMD is constant, as it remains the leading cause of irreversible blindness in individuals aged 60 years and older.1 Intermediate AMD is the stage prior to the late stage of AMD that is characterized by significant vision loss associated with the onset of GA or exudative AMD.2 iAMD is characterized by structural changes such as the accumulation of drusen and pigmentary abnormalities.
Although anti-VEGF therapy is effective in managing exudative neovascular AMD by controlling macular neovascularization and reducing exudation, it doesn’t address the underlying progression of nonexudative AMD toward GA.3 Similarly, emerging therapies such as complement inhibitors can modestly slow the rate of GA enlargement, but they aren’t curative and don’t reverse vision loss that has already occurred.4,5 These limitations underscore the urgency to identify therapies for iAMD to slow the progression to late AMD. The need to identify eyes with iAMD at high risk for progression to late-stage disease is obvious. Testing potential disease-modifying therapies in clinical trials can be completed within one to two years, rather than requiring five years of follow-up.
High-risk OCT biomarkers in iAMD
The development of optical coherence tomography has greatly improved our ability to visualize and quantify structural changes in the retina and choroid associated with the stages of AMD and serve as harbingers of disease progression. OCT biomarkers have become central to detecting high-risk features in iAMD that offer potential pathways for early intervention.6 Several OCT biomarkers have been proposed as reliable predictors of disease progression from iAMD to GA. These include the central macular drusen volume,7,8 the area of hyperreflective foci (HRF),9,10 the presence of calcified or hyporeflective-core drusen,11-13 vitelliform material,14 subretinal drusenoid deposits (also known as reticular pseudodrusen)15 and decreased perfusion in the macular choriocapillaris.16
Among these, central macular drusen volume remains a core biomarker. Advanced image segmentation algorithms now permit accurate, reproducible quantification of drusen volume using commercial spectral-domain OCT devices. Research by Yehoshua et al. and Abdelfattah et al. showed that a central drusen volume >0.03 mm³ was easily measured and could serve as an OCT biomarker that was associated with disease progression from iAMD to late exudative AMD.7,17
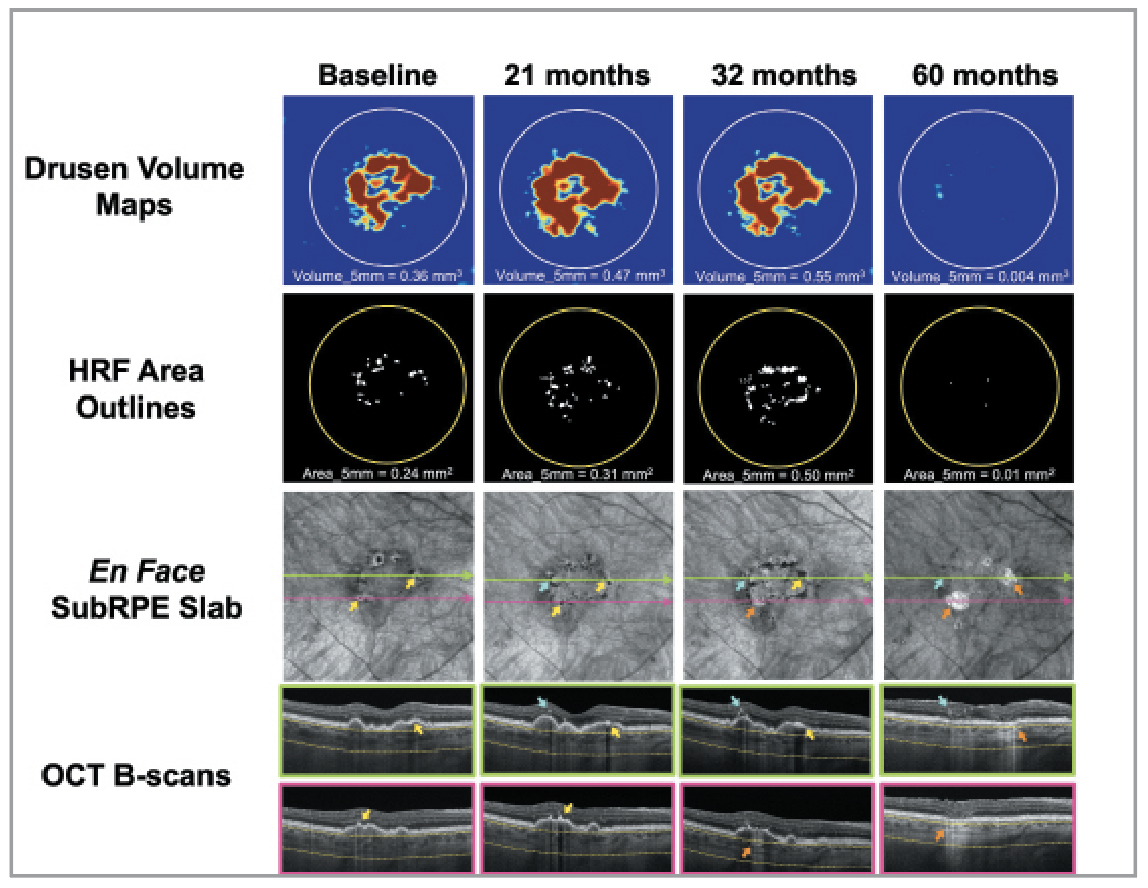 |
| Figure 1. Longitudinal SS-OCTA imaging from an iAMD eye with a large drusen volume and HRF located both intraretinally (blue arrows) and along the RPE (yellow arrows), developing large choroidal hyperTDs (orange arrows) over 60 months of follow-up. (Adapted and reprinted with permission from Berni A, Shen M, Cheng Y, et al. The total macular burden of hyperreflective foci and the onset of persistent choroidal hypertransmission defects in intermediate AMD. Am J Ophthalmol. 2024;267:61-75.) |
More recently, Liu et al.8 used swept-source OCT angiography and an automated algorithm for measuring drusen volume and identifying large hypertransmission defects (hyperTDs). Large hyperTDs represent an early structural sign of impending GA, and this OCT biomarker is now FDA-approved as a clinical trial endpoint for studying the progression from iAMD to GA. Large hyperTDs are defined as bright areas at least 250 µm in greatest linear dimension identified on en face sub-retinal pigment epithelium slabs with boundaries positioned 64 µm to 400 µm beneath Bruch’s membrane. The use of en face images derived from dense macular raster scans allows for the simultaneous evaluation of multiple biomarkers without the need to scroll through every B-scan. Using longitudinal data from a prospective cohort of iAMD eyes, the researchers8 used drusen volume to identify eyes at higher risk of disease progression and designed a clinical trial to test therapeutic strategies targeting hyperTD formation and growth.
Building upon this framework, Berni et al.9,10 proposed an alternative approach for stratifying risk by incorporating HRF into the risk assessment.
HRF are typically observed on OCT B-scans as discrete intraretinal lesions with reflectivity equal to or greater than that of the RPE band. These foci may correspond to different cell types or debris depending on the disease. In AMD, these HRF correspond to migrating RPE cells, activated microglia, lipid-laden macrophages or photoreceptor remnants. Moreover, in AMD, intraretinal HRF are frequently observed during progression from iAMD to both GA and exudative AMD. In dry AMD, iHRF likely represent RPE cells detaching and migrating from the monolayer, as supported by histologic and longitudinal imaging studies. However, not all HRF are intraretinal. Some remain confined to the RPE monolayer, referred to as rpeHRF. These rpeHRF are usually seen as areas of RPE thickening with increased reflectivity, and while not elevated above the monolayer, they’re considered important in predicting disease progression.
In longitudinal studies of iAMD, researchers observed that eyes could progress to hyperTDs even in the absence of a large drusen volume.9,10 Using the same SS-OCTA raster scans as used by Liu et al., they applied a semiautomated algorithm capable of identifying and quantifying the area of HRF based on their enhanced optical properties. While both iHRF and rpeHRF scatter both incident and reflected light and can be easily identified by detecting choroidal hypoTDs on the same en face subRPE slabs used to identify hyperTDs, Berni et al.9 were able to quantify the total area of HRF in the central macula, including both iHRF and rpeHRF. While the univariable analysis showed that the total HRF burden included both iHRF and rpeHRF predicted disease progression, the multivariable model revealed that only rpeHRF was the most predictive biomarker. However, for simplicity, when using a quantitative biomarker to predict disease progression, we propose that it’s easier to use the total HRF area as a practical metric for identifying high-risk eyes. Of note, measurements of the total macular HRF area outperformed drusen volume in predicting disease progression when both variables were included in a multivariable model. However, when considered separately, drusen volume still remained a valid marker of progression.
When separately considering drusen volume and the area of HRF, Berni et al.9 showed that eyes with a baseline HRF area ≥0.07 mm² had a 90-percent likelihood of developing a hyperTD within five years, compared to an 87-percent likelihood in eyes with drusen volumes of 0.22 mm³ or more. While both markers performed well, drusen volume has the advantage of being automatically measured using OCT software widely available in clinical settings. In contrast, HRF assessment still requires semi-automated segmentation and manual refinement, which can be more time-consuming and technically demanding. Thus, while HRF burden may offer stronger predictive accuracy in clinical trials, drusen volume still remains a pragmatic and accessible biomarker for large-scale screening in clinical practice.
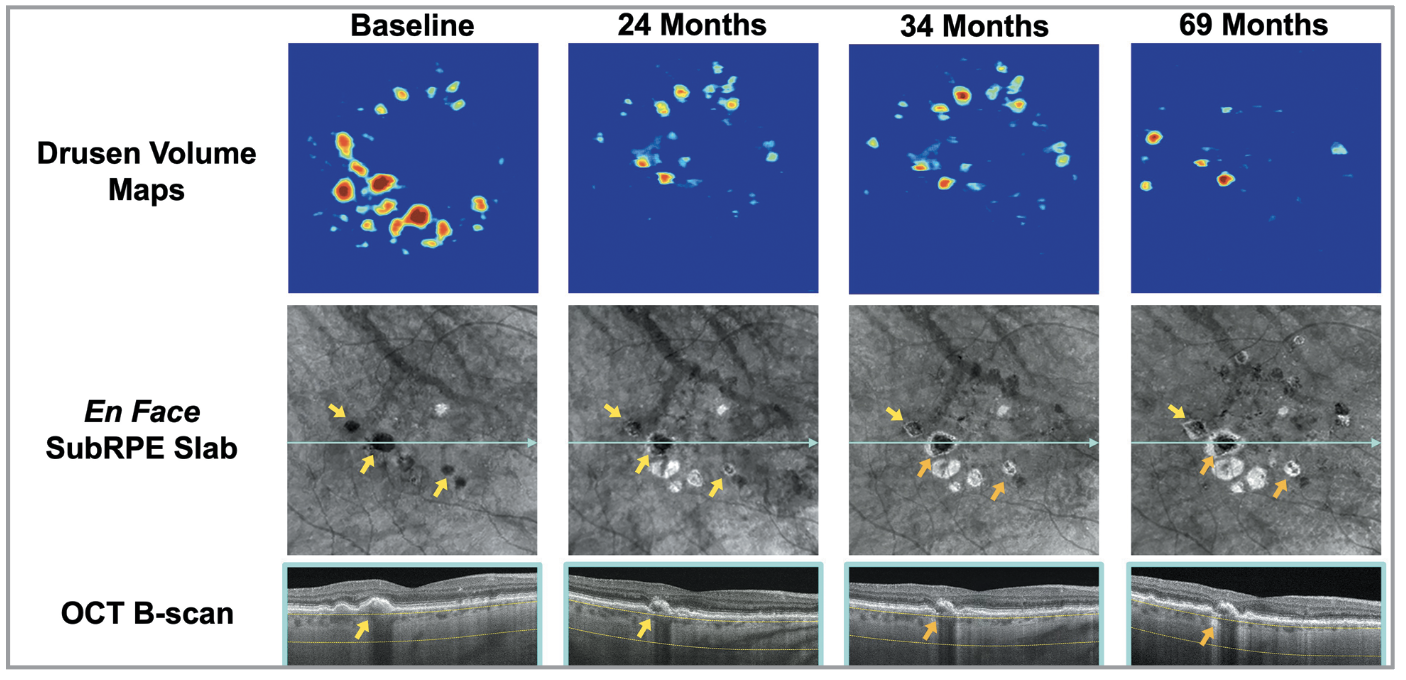 |
| Figure 2. Longitudinal SS-OCTA imaging from an iAMD eye with multiple calcified drusen evolving into large choroidal hyperTDs (orange arrows) over 69 months of follow-up. |
Calcified drusen are another clinical biomarker for AMD progression. CaD represent an advanced form of drusen degeneration and are considered robust OCT biomarkers of disease severity in iAMD. These lesions are easily recognized on structural OCT as highly reflective drusen, often with hyporeflective cores. On en face OCT, they produce choroidal hypoTDs similar to HRF, owing to their strong light-blocking properties, and they’re distinguished from HRF by reviewing the corresponding B-scans. Liu et al.12 first described this hallmark en face OCT appearance and linked CaD to the formation of large hyperTDs.
More recently, El-Mulki et al. (paper currently under revision) demonstrated that the mere presence of CaD, irrespective of their size or area, significantly elevates the risk of hyperTD development. As CaD often signify advanced pathological remodeling, eyes with these lesions may be less responsive to therapeutic intervention, further emphasizing their clinical relevance.
For this reason, identifying CaD at the time of clinical trial enrollment is crucial. Their presence may skew treatment outcomes if not appropriately accounted for, as they disproportionately elevate the baseline risk of progression. As a result, clinical studies should either ensure balanced distribution of CaD across study arms or consider excluding these eyes from early-phase intervention trials. While exclusion may reduce recruitment speed, it enhances study reliability and ensures a clearer assessment of therapeutic efficacy in treatable cases.
Bottom line
High-risk OCT biomarkers, particularly drusen volume, HRF and CaD, provide vital prognostic harbingers of progression from iAMD to GA. Their early identification through advanced imaging modalities like SS-OCTA enables timely intervention, personalized monitoring and improved clinical trial design. Continued research into OCT biomarkers will be instrumental in enhancing AMD management and preserving vision in at-risk populations. RS
REFERENCES
1. Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7:1:31.
2. Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:4:844-851.
3. Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:3:CD005139.
4. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402:10411:1434-1448.
5. Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402:10411:1449-1458.
6. Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on oct: Classification of atrophy report 3. Ophthalmology. 2018;125:4:537-548.
7. Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118:12:2434-2441.
8. Liu J, Shen M, Laiginhas R, et al. Onset and progression of persistent choroidal hypertransmission defects in intermediate age-related macular degeneration: a novel clinical trial endpoint. Am J Ophthalmol. 2023;254:11-22.
9. Berni A, Shen M, Cheng Y, et al. The total macular burden of hyperreflective foci and the onset of persistent choroidal hypertransmission defects in intermediate AMD. Am J Ophthalmol. 2024;267:61-75.
10. Berni A, Kastner JD, Shen M, et al. Hyperreflective foci along the retinal pigment epithelium predict the onset of large choroidal hypertransmission defects in age-related macular degeneration. Am J Ophthalmol. 2025;274:76-90.
11. Goh KL, Abbott CJ, Hadoux X, et al. Hyporeflective cores within drusen: association with progression of age-related macular degeneration and impact on visual sensitivity. Ophthalmol Retina. 2022;6:4:284-290.
12. Liu J, Laiginhas R, Shen M, et al. Multimodal imaging and en face OCT detection of calcified drusen in eyes with age-related macular degeneration. Ophthalmol Sci. 2022;2:2:100162.
13. Nittala MG, Corvi F, Maram J, et al. Risk factors for progression of age-related macular degeneration: population-based Amish Eye Study. J Clin Med. 2022;11:17:5110.
14. Mahmoudi A, Lindenberg S, Corradetti G, et al. Predictive factors influencing the evolution of acquired vitelliform lesions in intermediate age-related macular degeneration eyes. Ophthalmol Retina. 2024;8:9:863-871.
15. Hirabayashi K, Yu HJ, Wakatsuki Y, Marion KM, Wykoff CC, Sadda SR. OCT risk factors for development of atrophy in eyes with intermediate age-related macular degeneration. Ophthalmol Retina. 2023;7:3:253-260.
16. Corvi F, Tiosano L, Corradetti G, et al. Choriocapillaris flow deficits as a risk factor for progression of age-related macular degeneration. Retina. 2021;41:4:686-693.
17. Abdelfattah NS, Zhang H, Boyer DS, et al. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci. 2016;57:4:1839-1846.