| Eighth Annual Pipeline Report |
Take-home points
|
 |
How we compiled this listing This listing was compiled from company press releases and regulatory filings, published reports in the literature, searches on ClinicalTrials.gov and the European Union Clinical Trials Register (EurdraCT), presentations at Angiogenesis, Exudation and Degeneration 2024, the American Academy of Ophthalmology Retina Subspecialty Day, American Society of Retina Specialists, Retina Society, Association for Research in Vision and Ophthalmology, Retinal Cell and Gene Treatment Innovation Summit, EURETINA 2024 and OIS Retina 2024, supplemented with conversations and correspondence with a multitude of clinical investigators and representatives of trial sponsors. |
| List of tables Investigative agents for nAMD, DME, DR and RVO, page 14 Investigative programs in geographic atrophy and dry AMD, page 17 Gene therapy trials in AMD, DR and DME, page 19 Anti-VEGF biosimilars in clinical Investigative therapies for inherited retinal disease, page 24 Investigative device-based platforms, page 26 |
If 2023 was the Year of Geographic Atrophy, 2024 was the Year of the Biosimilar, specifically the aflibercept 2-mg biosimilar. The Food and Drug Administration approved five of them in 2024: Opuviz (Samsung Bioepis); Yesafili (Biocon Biologics); Ahzantive (Formycon); Enzeevu (Sandoz); and Pavblu (Amgen).
This year’s listing consists of 106 different investigative programs in retinal disease. Last year’s list topped out at 100.
Listings for inherited retinal disease and gene therapies for exudative retinal disease added the most new candidates with six each. The biosimilars listing had the largest contraction, deflating from 15 in 2024 to eight this year.
The only other exit from last year’s lists because of an FDA approval is the Valeda Light Delivery System (LumiThera), which appeared in the listing for devices.
This year’s lists reflect a number of other exits because the programs have either been abandoned or haven’t had information updates for more than a year, each of which is addressed under their respective categories. In the primary list, the following two candidates in 2024 were dropped from this year’s for lack of updated information:
- AKST4290 (Alkahest), an oral inhibitor treatment for diabetic retinopathy.
- Umedaptanib pegol (Ribomic), formerly RBM-007, which is still listed on the company’s website, but hasn’t had an update to its ClinicalTrials.gov listing since 2023.
By the same token, the primary listing includes three new programs, all of which are noted as such in the tabular listing and accompanying narrative.
A number of carryover listings appear under new names. The names under which these candidates appear this year and under which they previously appeared are:
- Axpaxli (Ocular Therapeutics) for OTX-TKI.
- Duravyu (EyePoint Pharmaceuticals) for EYP-190.
- Nesvategrast (OcuTerra Therapeutics) for OTT166.
- Sozinibercept (Opthea) for OPT-302.
- Xiflam (InflammX) for Tonabersat.
A few agents have also added new names. They include:
- AXT107 (AsclepiX Therapeutics) has added the new name gersizangitide.
- D-4517.2 (Ashvattha Therapeutics) has added the name migaldendranib.
- Restoret (Merck/Eyebiotech) now includes the name MK-3000.
- IBI302 (Innovent Biologics) carries the additional name of
Efdamrofusp alfa.
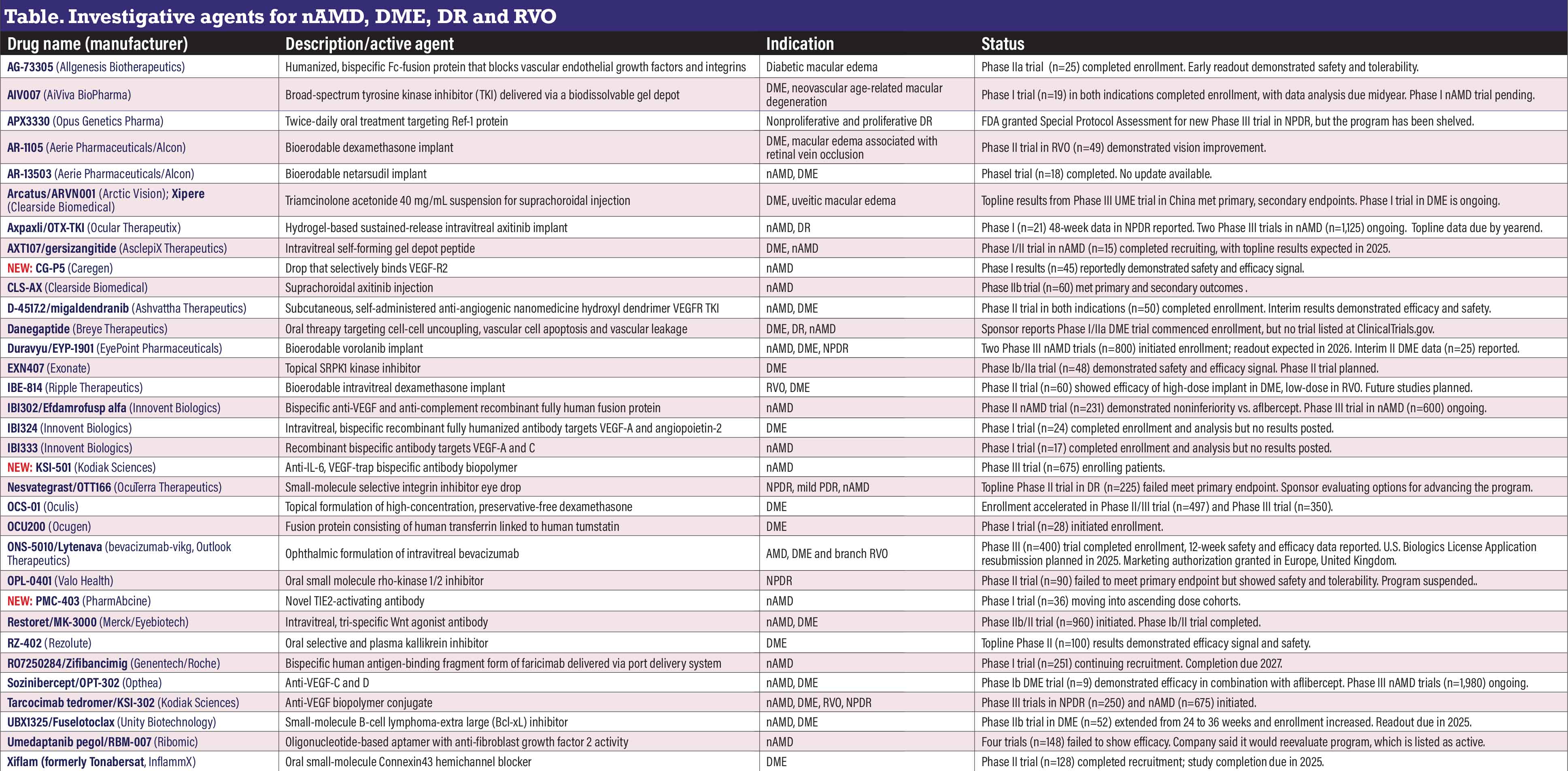
Investigative agents for nAMD, DME, DR and RVO
AG-73305 (Allgenesis Biotherapeutics)
This humanized, bispecific Fc-fusion protein for intravitreal injection aims to simultaneously block vascular endothelial growth factors and integrins. A Phase IIa trial (n=25, NCT05301751) in diabetic macular edema completed enrollment. Top-line results demonstrated safety and tolerability.
AIV007 (AiViva BioPharma)
This broad-spectrum tyrosine kinase inhibitor (TKI) for periocular administration forms a drug depot using proprietary JEL technology. The Phase I trial (n=19, NCT05698329) in DME and neovascular age-related macular degeneration has completed enrollment. Study completion is anticipated in the first quarter next year. Results of a separate feasibility Phase I trial in nAMD are pending (n=3, NCT04422899).
APX3330 (Opus Genetics)
APX3330 is an oral, small-molecule inhibitor of the transcription factor regulator Ref-1 that blocks downstream VEGF proliferation and inflammation. Ocuphire Pharma developed the agent, then acquired Opus Genetics last year. Opus said it would seek a strategic partner for APX-3330. The Phase II trial in nonproliferative diabetic retinopathy (n=103, NCT04692688) didn’t meet its primary endpoint, but the company did get a Special Protocol Assessment from the Food and Drug Administration for a Phase III trial in NPDR.
 |
| Click here to view the table in the digital edition. |
AR-1105, AR-13503 (Aerie Pharmaceuticals/Alcon)
While no updates have been posted for either candidate in the past year,
Alcon reports that both programs are still active. AR-1105 is a dexamethasone implant platform for DME and retinal vein occlusion. An open-label six-month study (n=49, NCT03739593) in patients with macular edema due to RVO demonstrated improved best-corrected visual acuity. AR-13503 is a rho-kinase inhibitor implant that showed efficacy as a monotherapy and anti-VEGF adjunct in preclinical studies. A Phase I trial in nAMD and DME has been completed, but no results have been posted (n=18, NCT03835884).
Arcatus/ARVN001 (Arctic Vision); Xipere (Clearside Biomedical)
Arcatus, a triamcinolone acetonide 4-mg/0.1-mL injection suspension vial kit for suprachoroidal injection, received regulatory approval in Australia and Singapore for the treatment of uveitic macular edema, an indication for which the Clearside iteration, Xipere, is approved in the United States. Last year Arctic Vision reported positive top-line results from its Phase III clinical trial of Arcatus, also known as ARVN001, for UME in China. Clearside lists on its website a Phase I trial in DME as active in Asia outside Japan, but none is registered with ClinicalTrials.gov.
Axpaxli/OTX-TKI (Ocular Therapeutics)
Patients have been randomized in the SOL-1 Phase III trial in nAMD (n=300, NCT06495918) of this intravitreal implant using axitinib, a TKI, which is becoming an emerging class for treating exudative retinal disease. The trial is running alongside the registrational Phase III SOL-R trial (n=825, NCT06495918) in nAMD. The SOL-1 trial is due to report top-line data later in the year, Ocular states.
Enrollment has been completed in the Phase Ib HELIOS trial in moderately severe-to-severe NPDR (n=21, NCT05695417). Meanwhile, Ocular says it plans to seek FDA feedback on the clinical trial design for further study in NPDR.
AXT107/gersizangitide (AsclepiX Therapeutics)
AXT107 is a microparticulate suspension for intraocular injection that targets VEGF receptor-2 and activates the vessel stabilizing receptor tyrosine kinase (Tie2). AxclepiX completed recruiting for a Phase I/IIa DISCOVER trial in nAMD (n=15, NCT05859776) that’s evaluating 40-week outcomes of three dose levels of a single injection of AXT107.
NEW: CG-P5 (Caregen)
South Korea-based Caregen has reported interim Phase I results from a U.S. trial of CG-P5 (n=45, NCT06132035), an eye drop that selectively binds to VEGFR-2. Results demonstrated improvement in BCVA and central retinal thickness, along with strong safety and tolerability profiles, Caregen reports. The company plans to expand indications in Phase II trials to include DR and other related retinal conditions.
CLS-AX (Clearside Biomedical)
This suprachoroidal suspension of the TKI axitinib is the focus of the Phase IIb ODYSSEY trial (n=60, NCT05891548) in nAMD, with
aflibercept as the comparator. The trial met its primary and secondary endpoints. CLS-AX patients had stable BCVA and CST for up to six months vs. aflibercept. CLS-AX also demonstrated satisfactory safety to week 36, including mandatory redosing of CLS-AX at week 24. No patients required additional dosing at up to 12 weeks, and 67 percent didn’t need any before mandatory redosing at week 24.
D-4517.2/migaldendranib (Ashvattha Therapeutics)
Now also known as migaldendranib, D-4517.2 is a TKI that targets
VEGFR. It’s intended for at-home, subcutaneous administration every two or four weeks for up to 40 weeks after a single intravitreal injection of aflibercept 2 mg. Interim 24-week results from the Phase II trial in nAMD and DME (n=50, NCT05387837) showed a reduction in supplemental aflibercept, along with maintenance of BCVA and sustained reductions in subretinal fluid.
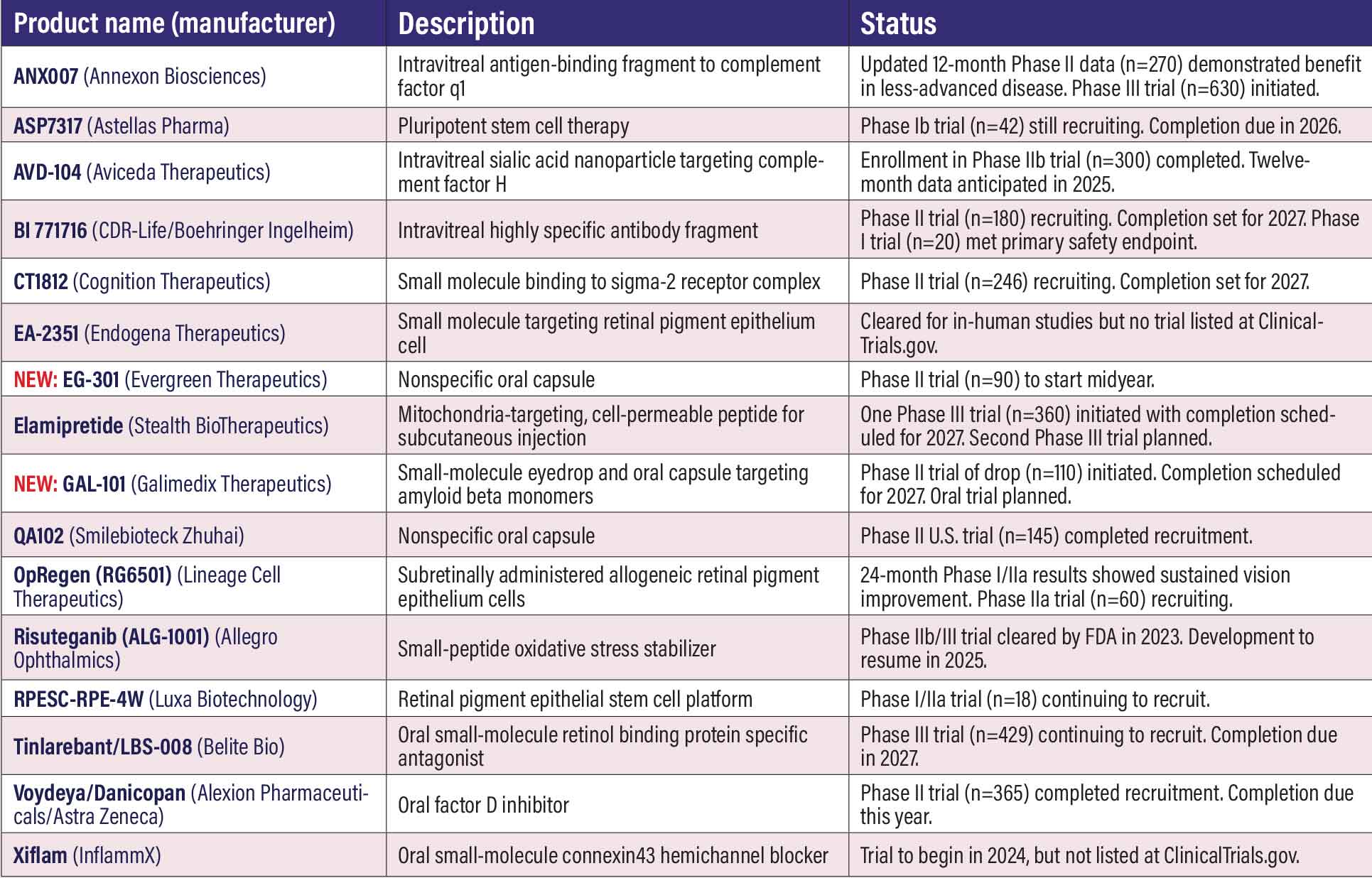
Clinical trials in geographic atrophy, dry AMD This year’s listing consists of 16 candidates, the same as last year, but with two new additions and two subtractions. Removed from list are: IONIS-FB-LRx (Ionis Pharmaceuticals). The company discontinued the program. ADX-248 (Aldeyra Therapeutics). This appears in the main listing, but the Investigational New Drug Application the company said it would file last year for dry age-related macular degeneration in patients with dark-adaptation deficit didn’t materialize. There has been one name change: Voydeya (Alexion) was formerly listed under Danicopan. ANX007 (Annexon Biosciences). This intra- ASP7317 (Astellas Pharma). ASP7317 aims to replace lost retinal pigment epithelium cells with new cells generated from pluripotent stem cells. A Phase Ib trial (n=42, NCT031781149) is recruiting. AVD-104 (Aviceda Therapeutics). This intravitreal glycan (sialic acid) nanoparticle targets the self-pattern recognition receptors on overly activated retinal macrophages and microglia. It’s designed to repolarize these immune cells to reduce inflammation while enhancing complement factor H activity. The Phase IIb SIGLEC trial in GA (n=300, NCT05839041) completed enrollment. BI 771716 (CDR-Life/Boehringer Ingelheim). The first patient was dosed last year in the Phase I trial in GA (n=20, NCT06006585), with completion set for midyear. The Phase I trial in GA (n=20, NCT06006585), which completed recruitment last year, met its primary safety endpoint, but specific results haven’t been disclosed. BI 771716 is a highly specific antibody fragment designed to penetrate all layers of the retina. The Phase II VERDANT trial (n=180, NCT06722157) is recruiting. CT1812 (Cognition Therapeutics). This small-molecule oral agent is designed to penetrate the blood-brain and blood-retina barriers and bind selectively to the sigma-2 receptor complex, which has been implicated in oxidative stress that causes loss of function in RPE cells. Enrollment in the Phase II MAGNIFY trial in GA (n=246, NCT05893537) is continuing. EA-2351 (Endogena Therapeutics). This small molecule targets RPE cells to regenerate and restore photoreceptor function and reduce GA lesion size. The FDA last year cleared an Investigational New Drug application for a Phase Ib trial, but it has not yet been listed at ClinicalTrials.gov. NEW: EG-301 (Evergreen Therapeutics). This unspecified oral agent for rapidly progressing dry AMD aims to enhance the anti-inflammatory and antioxidative function of RPE cells, improve autophagosome transport function and inhibit complement activation of epithelial cells, and protect mitochondria. A Phase II trial in GA will start this year (n=90, NCT05170048). Elamipretide (Stealth BioTherapeutics). The Phase III ReNew trial in dry AMD (n=360, NCT06373731) opened enrollment last year. A second Phase III trial, ReGAIN, not yet listed at ClinicalTrials.gov, will also evaluate Elamipretide, a cell-permeating peptide that has demonstrated properties to normalize mitochondrial structure in dry AMD. NEW: GAL-101 (Galimedix Therapeutics). GAL-101 is a small-molecule eyedrop targeting misfolded amyloid-β (Aβ) monomers to prevent formation of toxic Aβ oligomers and protofibrils. A Phase II trial in GA (n=110, NCT06659549) started recruiting last year. Galimedix also says it started a Phase I study of an oral formulation, but no such trial is listed at ClinicalTrials.gov. QA102 (Smilebiotek Zhuhai). QA102 is an oral capsule, but no further description is available and the sponsor doesn’t have a website. A Phase II U.S. trial (n=50, NCT 05536752) last year completed recruitment. The company posted an update with ClinicalTrials.gov early this year. OpRegen/RG6501 (Lineage Cell Therapeutics). Two-year results from the Phase I/IIa trial (n=24, NCT02286089) demonstrated sustained visual and anatomical improvements, along with extensive bleb coverage of the GA area.1 A Phase IIa study is recruiting patients (n=60, NCT05626114). OpRegen is a suspension of human allogeneic RPE cells delivered subretinally. Risuteganib/Luminate (Allegro Ophthalmics). Allegro says it plans to move forward on the Phase IIb/III clinical trial in intermediate dry AMD for this small-peptide oxidative stress stabilizer. No trial is registered at ClinicalTrials.gov. RPESC-RPE-4W (Luxa Biotechnology). RPESC-RPE-4W is derived from adult RPE stem cells (RPESC). The Phase I/IIa trial (n=18, NCT04627428) is recruiting. Luxa last year obtained a $4 million grant to support the trial. Tinlarebant/LBS-008 (Belite Bio). This oral agent targets vitamin A-based toxins, known as bisretinoids, which may contribute to GA progression. The Phase III PHOENIX clinical trial (n=429, NCT05945993) is recruiting patients. A Phase III trial in Stargardt disease is ongoing. Voydeya/Danicopan (Alexion Pharmaceuticals/AstraZeneca). Also known as ALXN2040, Voydeya is an oral factor D inhibitor being developed as an add-on to complement factor 5-inhibition therapies for patients with paroxysmal nocturnal hemoglobinuria. Alexion is also evaluating Voydeya as a monotherapy for GA in a Phase II trial (n=365, NCT05019521). The trial completed enrollment last year. Voydeya has been granted Breakthrough Therapy designation by the Food and Drug Administration. Xiflam (InflammX). Xiflam is an oral small molecule that targets the Connexin43(Cx43) hemichannel protein. The FDA in 2023 cleared a trial in early stage dry AMD, but no trial has been posted at ClinicalTrials.gov. The company still mentions Xiflam in its filings.
REFERENCE Telander D. Untitled presentation at 2024 Retinal Cell & Gene Therapy Innovation Summit. May 6, 2024. |
Danegaptide (Breye Therapeutics)
This oral treatment for DR and DME targets cell-to-cell uncoupling, vascular cell apoptosis and vascular leakage in early disease. Breye last year said it initiated a Phase Ib/IIa trial in DME, but the only trial listed at ClinicalTrials.gov is in cardiovascular disease.
Duravyu/EYP-1901 (EyePoint Pharmaceuticals)
This TKI candidate uses vorolanib in the bioerodable sustained-release insert with the Durasert E platform. Six-month results from the ongoing Phase II VERONA trial in DME (n=25, NCT06099184) demonstrated extended time to the first supplemental injection compared to aflibercept controls, along with continued safety. The 2.7-mg formulation demonstrated a 7.1-letter gain in BCVA and 76-mm reduction in CST at week 24. The supplement-free rate was 73 percent vs. 50 percent for aflibercept.
Completion of enrollment in the Phase III LUGANO (n=400, NCT06668064) and LUCIA (n=400, NCT06683742) pivotal trials in nAMD is expected later this year.
EXN407 (Exonate)
This twice-daily eyedrop aims to inhibit the serine-arginine protein kinase 1 enzyme, overexpression of which has been implicated in cancer, and modulate the downstream effects of VEGF. In a Phase Ib/IIa trial (n=48, NCT0456756) in DR and DME, 60 percent of treated patients with mild NPDR had decreased vascular leakage vs. 20 percent of placebo patients. A Phase II trial is pending.
IBE-814 (Ripple Therapeutics)
This bioerodable intravitreal implant delivers low-dose dexamethasone. The Phase II trial in DME and RVO (n=60, NCT04576689) evaluated low-dose (one 70-mg implant) and high-dose (two implants) treatments. In DME, the low-dose group had a –68 mm improvement in CST and a 1.9-letter loss in BCVA; the high-dose group had a –94 mm improvement and an 8.7-letter gain at nine months. In RVO, the low-dose arm had superior outcomes.1 Ripple says it will pursue future studies of the high-dose implant in DME and low-dose in RVO.
IBI302/efdamrofusp alfa (Innovent Biologics)
IBI302 is an intravitreal bispecific
antibody that targets both VEGF and complement factor 3b/4b. A Phase II trial in nAMD (n=132, NCT05403749) randomized patients to three arms: IBI302 6.4 mg; IBI302 8 mg; and aflibercept 2 mg. After loading doses, IBI302 recipients were dosed at adjusted intervals of every eight or 12 weeks; aflibercept patients every eight weeks. BCVA gains in the IBI302 arms were noninferior at week 40. Eighty-one percent of the low-dose and 88 percent of the high-dose arms were dosed every 12 weeks. A Phase III trial in nAMD (n=400, NCT05972473) is recruiting.
IBI 324, IBI333 (Innovent Biologics)
IBI324 is an intravitreal, dual-target specific recombinant fully humanized antibody that targets VEGF-A as well as angiopoietin-2 (Ang-2). The Phase I dose-escalation trial in DME (n=24, NCT05489718) was completed last year, as was the Phase I trial in nAMD (n=17, NCT05639530), but the trial sponsor hasn’t reported any results.
NEW: KSI-501 (Kodiak Sciences)
Also known as tabirafusp tedromer, KSI-501 is described as a first-in-class anti-interleukin-6, VEGF-trap bispecific antibody biopolymer conjugate. It’s being evaluated in a Phase III trial in nAMD alongside tarcocimab tedromer (n=675, NCT06556368).
Nesvategrast/OTT166 (OcuTerra Therapeutics)
Top-line results from the Phase II trial in DR (n=225, NCT05409235) failed to demonstrate a statistically significant improvement with this topical small-molecule, selective integrin inhibitor. The Phase II DR:EAM trial (Diabetic Retinopathy: Early Active Management) enrolled adults with moderately severe-to-severe NPDR or mild PDR and minimal vision loss. The trial met the primary safety endpoint. OcuTerra says it’s exploring strategic options to advance the program.
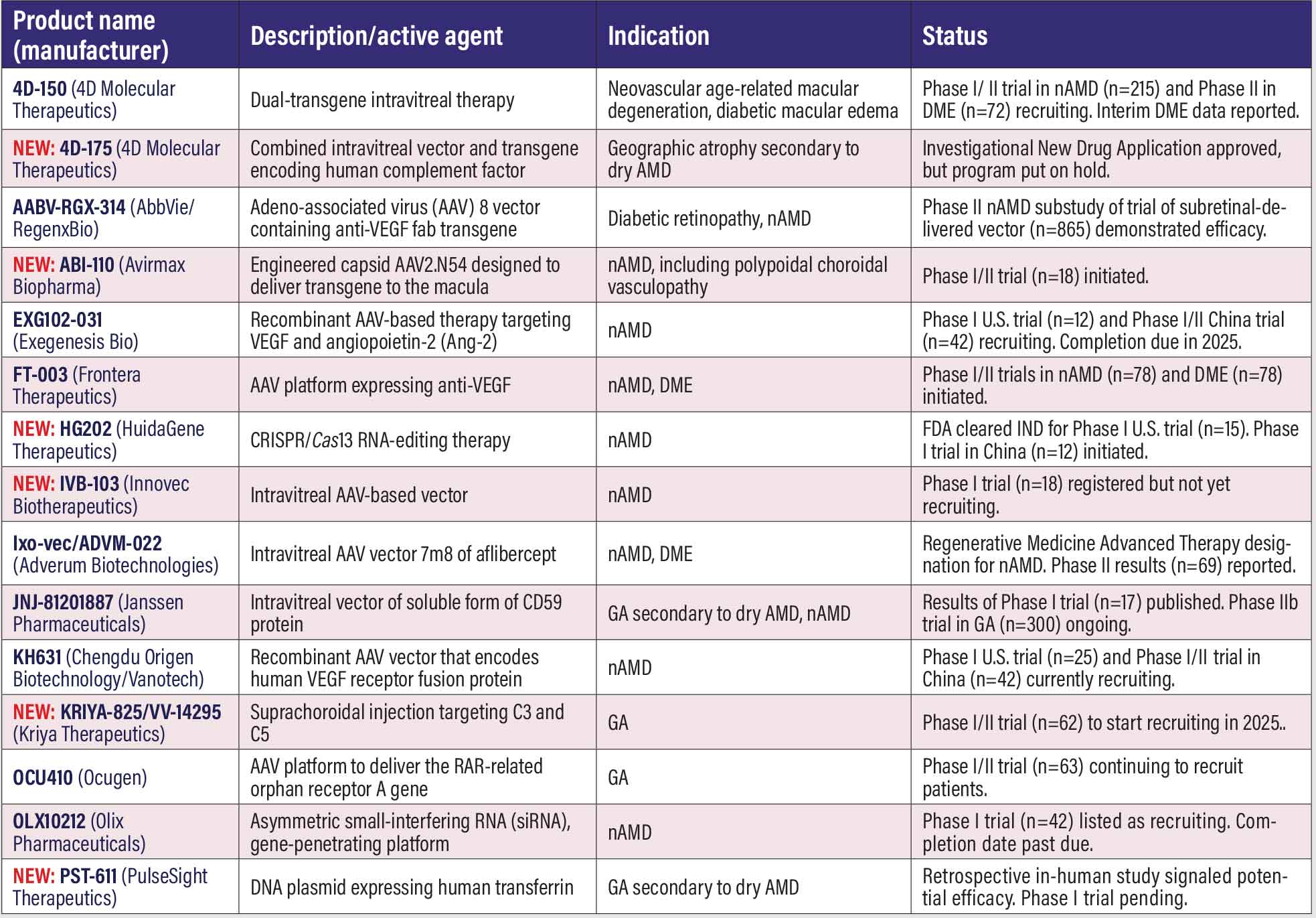
Gene therapy candidates for AMD, DR, DME This list has seen the largest proportional growth of all listings, from 10 in 2024 to 15 this year. One candidate has been dropped. No updated information on IBI324 (Innovent Biologics) has been posted for well over a year. However, six new candidates, as noted, have been added. 4D-150 (4D Molecular Therapeutics). Supplemental data from the Phase II PRISM trial (n=150, NCT05197270) evaluating 4D-150 in neovascular age-related macular degeneration found a single intravitreal dose of 3x1010 vector genes per eye (vg/eye) resulted in sustained reduction and stabilization of mean central subfield thickness vs. aflibercept at all time points. The trial focused on a subgroup of patients who didn’t need supplemental aflibercept in addition to the 4D-150 treatment. In this subgroup, the mean difference vs. aflibercept in CST change from baseline for weeks 20 and 24 was -31 mm in favor of 4D-150. 1 Earlier this year, 4DMT reported that top-line interim data from the first stage of the Phase II SPECTRA trial in diabetic macular edema (n=72, NCT05930561) demonstrated signals of clinical activity with the 3x1010 vg/eye dose, with a sustained gain of best-corrected visual acuity of 8.4 letters and a reduction of CST of -194 mm through week 32. The company said it also reached agreement with the Food and Drug Administration on a registrational pathway in DME. 4D-150 consists of the targeted and evolved intravitreal vector, R100, and a dual transgene payload that expresses aflibercept and a vascular endothelial growth factor-C RNA interference to inhibit VEGF A, B, C and placental growth factor for low-dose intravitreal delivery. NEW: 4D-175 (4DMT). The FDA cleared the Investigational New Drug Application for 4D-175, another R100 vector-based intravitreal therapy, this one for geographic atrophy, but earlier this year 4DMT said it would put the program on hold. AABV-RGX-314 (RegenxBio/AbbVie). AABV-RGX-314 is an adeno-associated virus-8 vector that contains an anti-VEGF transgene. The sponsors report they expect data from the Phase II/III ATMOSPHERE (n=540, NCT04704921) and In nAMD, nine-month data from the Phase II fellow eye substudy of subretinal ABBV-RGX-314 in patients with bilateral disease (n=865, NCT03999801), reported a 97 percent reduction in annualized anti-VEGF treatment burden, with 78 percent of patients not needing any injections.2 None of the patients required two or more supplemental injections. NEW: ABI-110 (Avirmax Biopharma). The first patient was dosed in the Phase I/IIa clinical trial in nAMD, including polypoidal choroidal vasculopathy (n=18, NCT06550011). ABI-110 uses the engineered capsid AAV2.N54 to deliver a therapeutic transgene to the macula. EXG102-031 (Exegenesis Bio). EXG102-031 is an injectable recombinant AAV-based gene therapy that aims to express a therapeutic fusion protein that binds to or neutralizes all known subtypes of VEGF and angiopoietin-2 (Ang-2). Two separate clinical trials in nAMD have been recruiting patients: a Phase I U.S. trial known as Everest (n=12, NCT05903794); and a Phase I/II trial in China (n=42, NCT06183814). FT-003 (Frontera Therapeutics). FT-003 is a recombinant AAV vector that targets retinal cells to express anti-VEGF. Frontera last year received FDA clearance for two Phase II trials in DME (n=78, NCT06492876) and nAMD (n=78, NCT06492863). The first patients were dosed last year in a Phase I trial in central involvement DME in China (n=18, NCT05916391). A Phase I trial in nAMD (n=18, NCT05611424) hasn’t yet started recruitment. NEW: HG202 (HuidaGene Therapeutics). The FDA cleared the IND filing for this CRISPR/Cas13 RNA-editing therapy for nAMD. Preclinical studies showed HG202 reduced choroidal neovascularization area by 87 percent, which HuidaGene reports was superior to both anti-VEGF antibodies and AAV-anti-VEGF gene therapy. An early Phase I trial in China (n=12, NCT06031727) is recruiting. The Phase I U.S. trial (n=15, NCT06623279) is to start recruiting this year. NEW: IVB-103 (Innovec Biotherapeutics/uBriGene Biosciences). The partners received FDA clearance for their IND application for this AAV platform designed as a single intravitreal injection for nAMD. Few details are available. The Phase I trial in nAMD (n=18, NCT06737354) is registered at ClinicalTrials.gov, but it hasn’t started recruiting. Ixo-vec/ADVM-022 (Adverum Biotechnologies). Ixo-vec is short for ixoberogene soroparvovec, an AAV vector that aims to induce expression of aflibercept. The FDA granted Regenerative Medicine Advanced Therapy designation for intravitreal Ixo-vec for treatment of nAMD. Top-line 52-week results from the LUNA Phase II trial in nAMD (n=69, NCT05536973) showed that both doses (6x1010 and 2x1011 vg/eye), maintained visual and anatomic endpoints for BCVA and CST, and resulted in treatment burden reductions of 88 and 92 percent, respectively. Four-year Phase I OPTIC top-line results of the 2x1011 dose (n=30, NCT03748784) demonstrated an 84-percent reduction in treatment burden, with nearly 50 percent of patients not needing additional injections. Ixo-vec is also the subject of a Phase II extension trial in DME (n=22, NCT05607810), which is a follow-on to the completed Phase II INFINITY trial (n=36, NCT04418427). JNJ-81201887 (Janssen Pharmaceuticals). This intravitreal vector aims to boost expression of soluble CD59, which inhibits formation of the membrane attack complex. Results from the Phase I study in GA secondary to dry AMD (n=17, NCT03144999) demonstrated slowed lesion growth over 24 months in the high-dose cohort (3.56x1011 vg/eye), and five of 17 patients had mild ocular inflammation.3 The Phase II trial in GA (n=300, NCT06635148) is recruiting. A Phase I study in nAMD (n=25, NCT03585556) has been completed since 2022 but no update has been posted. KH631 (Chengdu Origen Biotechnology/Vanotech). KH631 is a recombinant AAV vector that encodes a human VEGF receptor fusion protein. The Phase I U.S. trial (n=25, NCT05657301) and a Phase I/II trial in China (n=42, NCT05672121), both in nAMD, are currently recruiting patients. NEW: KRIYA-825/VV-14295 (Kriya Therapeutics). KRIYA-825 is an AAV vector that aims to express a novel complement receptor 2-complement receptor 1 (CR2-CR1) fusion protein to inhibit complement factors 3 and 4 in GA. It’s designed to be administered through a one-time in-office suprachoroidal injection. A Phase I/II trial (n=62, NCT06765980) is slated to start this year. OCU410 (Ocugen). Dosing in the high-dose cohort of the Phase I/II ArMaDa trial in GA (n=63, NCT06018558) was completed last year. The high dose is 200 µL of 1.5x1011 vg/mL of OCU410, also known as AAV-RORA, which uses an AAV platform to deliver the RORA (RAR-related orphan receptor A) gene to the retina to reduce lipofuscin deposits and oxidative stress. OLX10212 (Olix Pharmaceuticals). Completion of the Phase I trial in nAMD trial (n=42, NCT05643118) is past due. Early results previously demonstrated no serious safety signals. OLX10212 uses an asymmetric small-interfering RNA (siRNA), gene-penetrating technology to target genetic origins of inflammation. NEW: PST-611 (PulseSight Therapeutics). A clinical trial authorization has been submitted in France for this treatment for dry AMD and GA. PST-611 is a DNA plasmid expressing human transferrin, a desirable target in dry AMD.4 PulseSight says it anticipates starting the trial in the second quarter this year.
REFERENCES Maturi RK. Phase 2 population extension cohort in the PRISM trial evaluating 4D-150 in adults with neovascular age-related macular degeneration. Paper presented the American Society of Retina Specialists Annual Scientific Meeting. Stockholm, Sweden; July 17, 2024. Khanani AM. Subretinal delivery of investigational AABV-RGX-314 as a gene therapy for nAMD; First time results of a fellow eye bilateral dosing study. Paper presented at the American Academy of Ophthalmology Retina Subspecialty Day 2024. Chicago, IL; October 19, 2024. Heier JS, Cohen MN, Chao DL, et al. Phase 1 study of JNJ-81201887 gene therapy in geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2024;131:1377-1388. Bordet T, Youale J, Bigot K, et al. Transferrin is a drug candidate for the treatment of geographic atrophy (GA)/dry age-related macular degeneration (AMD). Poster presented at European Association for Vision and Eye Research 27th EVER Congress 2024. Paris; Nov. 6, 2024. |
OCS-01 (Oculis)
This topical form of preservative-free dexamethasone 15-mg/mL is the focus of the Phase II/III DIAMOND-1 trial (n=497, NCT05066997) and the Phase III DIAMOND-2 trial (n=350, NCT06172257). Oculis says it has accelerated enrollment in both trials.
OCU200 (Ocugen)
Ocugen describes OCU200 as a recombinant fusion protein that consists of tumstatin, an anti-inflammatory, anti-VEGF agent that binds to integrin receptors; and transferrin, which directs the drug to the choroid and retina. The Phase I trial in DME (n=28, NCT05802329) started this year.
ONS-5010/Lytenava/bevacizumab-vikg (Outlook Therapeutics)
This ophthalmic formulation of bevacizumab has been approved in the European Union and United Kingdom for nAMD, but still faces regulatory hurdles in the United States. Outlook reports the analysis of the full 12-week safety and efficacy results for the Phase III NORSE EIGHT trial in nAMD (n=400, NCT06190093) has been completed. At 12 weeks, ONS-5010 1.25 mg demonstrated a mean change in BCVA of +5.5 letters vs. +6.5 letters for ranibizumab. Outlook says it plans to resubmit the Biologics License Application (BLA) in the next few months. The FDA in 2023 rejected the first BLA.
OPL-0401 (Valo Health)
This oral, small-molecule, rho-kinase 1/2 inhibitor is the subject of the Phase II Spectra trial in patients with mild, moderate and severe NPDR (n=114, NCT05393284). Top-line data showed OPL-0401 failed to meet its primary or secondary endpoints, including efficacy vs. placebo in improving Diabetic Retinopathy Severity Score. No treatment-related adverse events were reported. Valo says it’s suspending the program while it seeks a development partner.
NEW: PMC-403 (PharmAbcine)
PMC-403 is a TIE2-activating antibody that’s in a Phase I trial in nAMD (n=36, NCT05953012) enrolling nonresponders to anti-VEGF therapy. The trial has moved into the fourth single-dose group of 4-mg and first multiple-dose group of 3-mg dosing.
Restoret/MK-3000 (Merck/Eyebiotech)
Merck last year acquired Eyebiotech and its lead candidate, Restoret, and added the additional label MK-3000. Restoret is an intravitreal, trispecific agonist antibody targeting the Wnt signaling pathway to halt vascular leakage and restore and maintain the blood-retinal barrier.
Merck last year initiated enrollment in the Phase IIb/III BRUNELLO trial in DME (n=960, NCT06571045). Trial completion is set for 2027. The Phase Ib/II AMARONE trial (it stands for Anti-permeability Mechanism and Age Related Ocular Neovascularization Evaluation) in DME and nAMD (n=33, NCT005919693) provided the basis for moving onto the Phase II/III trial, Eyebiotech reports.
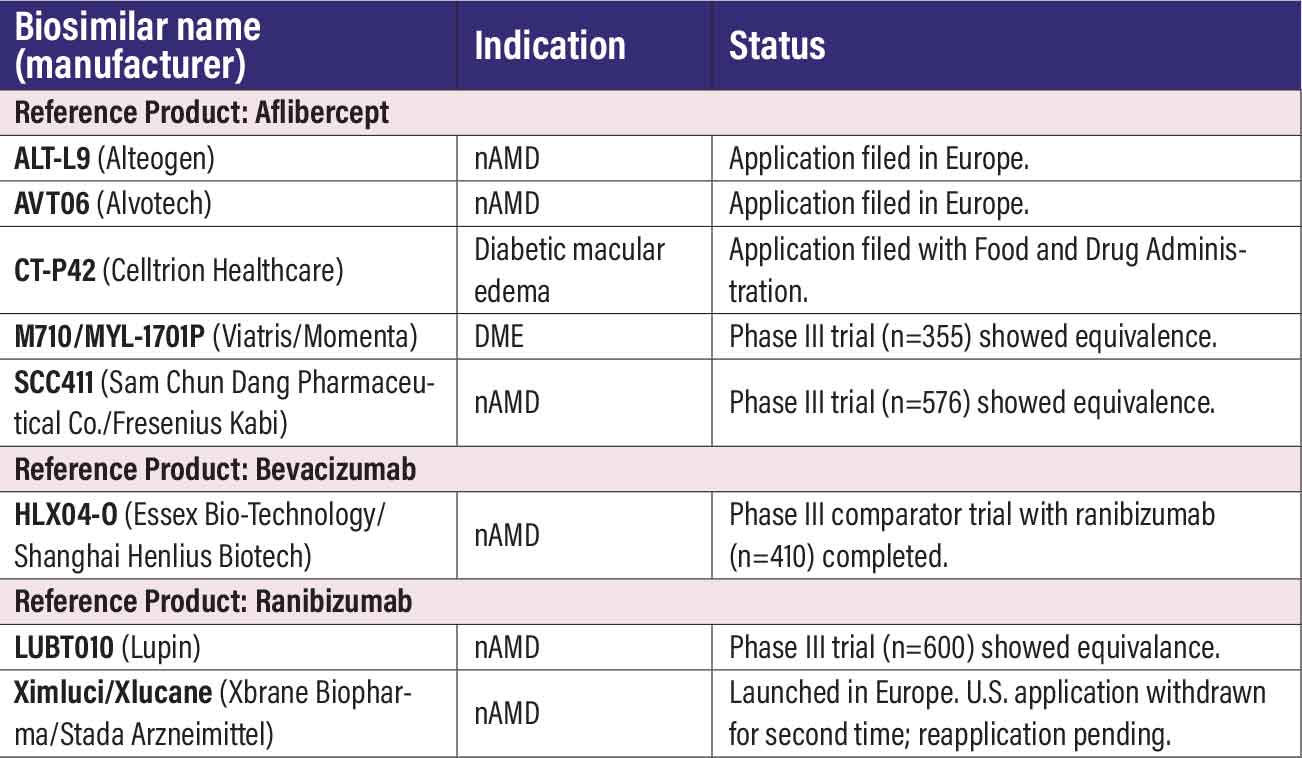
Anti-VEGF biosimilars in clinical trials Last year 15 biosimilars candidates were listed. This years’ list includes eight. Five previously listed aflibercept biosimilars were removed because they obtained Food and Drug Administration approval. One other biosimilar was removed from the aflibercept listing: LY9004 (Ocumension Therapeutics/Shandong Boan Biological Technology). No trial has been registered at ClinicalTrials.gov or the European Union Clinical Trials Register. The ranibizumab biosimilar CKD-701 (Chong Kun Dang Pharmaceutical) had been removed. It obtained approval in South Korea, and while the sponsor has partnerships with Pfizer and Amgen, no clinical trials are listed at ClinicalTrials.gov. AFLIBERCEPT BIOSIMILARS ALT-L9 (Alteogen). ALT-L9 is the subject of a Marketing Authorization Application (MAA) filed with the European Medicines Agency (EMA). The Phase III trial (n=413, EudraCT: 2021-004530-11) showed therapeutic equivalence with Eylea. AVT06 (Alvotech). Alvotech early last year reported positive top-line results from the Phase III ALVOEYE trial in neovascular age-related macular degeneration (n=413, NCT05155293). The study met its primary endpoint, with results demonstrating therapeutic equivalence and comparable safety with the reference produce. The EMA last summer accepted its MAA application. CT-P42 (Celltrion). EMA’s Committee for Medicinal Products for Human Use has adopted positive opinions and recommended marketing authorizations for CT-P42, also known as Eydenzelt. Indications are nAMD, macular edema following retinal vein occlusion, diabetic macular edema, and myopic choroidal neovascularization. An FDA application is pending. MYL-1701P (Biocon Biologics). Biocon took over this program, also known as M710, last year after it completed the integration of Viatris’ biosimilars portfolio. Results from a 52-week clinical trial in DME (n=355, NCT03610646) demonstrated equivalency to aflibercept.1 SCD411 (Sam Chun Dang Pharmaceutical Co./Fresenius Kabi). The Phase III trial in nAMD (n=576, NCT04480463) demonstrated equivalence with aflibercept in visual and safety outcomes.2 Sam Chun Dang Pharmaceutical last year entered into a licensing agreement with Germany-based Fresenius Kabi for global commercialization of SCD411. BEVACIZUMAB BIOSIMILAR HLX04-O (Essex Bio-Technology/Shanghai Henlius Biotech). The last patient visit had been completed in the Phase III trial in nAMD (n=410, NCT04740671) in the European Union and Australia, while a second Phase III trial in China (n=388, NCT05003245) is listed as status unknown. The biosimilar is approved in China for cancer indications. RANIBIZUMAB BIOSIMILARS LUBT010 (Lupin). Listed last year as “unknown status” (n=600, NCT04690556), the trial posting has since been updated and the trial itself completed, the trial sponsor reported last summer. The study met its primary endpoint of therapeutic equivalence in visual acuity improvement with Lucentis. Lupin has been marketing the ranibizumab biosimilar in India since 2022. XSB-001/Ximluci/Xlucane (Xbrane Biopharma/Valorum Biologics/STADA Arzneimittel). Launched in 2023 in Europe as Ximluci, the version known as Xbrane in the United States has had a rocky go with the FDA. After STADA Arzneimittel withdrew the Biologic Licensing Application filed in 2022, it resubmitted the BLA in 2023. But the FDA last April, just as the application’s Biosimilar User Fee Amendment date arrived, issued a Complete Response Letter. Xbrane and STADA then entered into a U.S. marketing agreement with Valorum Biologics. Xbrane last year said it would resubmit the BLA later in 2025, but no update is available. The Phase III trial in nAMD (n=582, NCT03805100) demonstrated equivalency with the reference product.
REFERENCES Bressler SB, Barve A, Ganapathi PC, et al. Aflibercept biosimilar MYL-1701P vs reference aflibercept in diabetic macular edema. JAMA Ophthalmol. 2024;142:952-960. Kang SW, Choi J, Sheth VS, et al. Comparison of the efficacy and safety of SCD411 and reference aflibercept in patients with neovascular age-related macular degeneration. Sci Rep. 2024;14:14752. |
RZ-402 (Rezolute)
This is an oral selective and plasma kallikrein inhibitor. Top-line results from the Phase II trial in DME (n=100, NCT05712720), enrolling patients who are either treatment-naive or had limited exposure to anti-VEGF, showed CST improved by around -50 mm at all three dose levels (50, 20 and 400 mg) compared to placebo, and by around ‑75 mm at the 200-mg dose at 12 weeks.
RO7250284/Zifibancimig (Genentech/Roche)
Zifibancimig is a bispecific human Fab form of faricimab delivered via the port-delivery implant used with Susvimo. The three-part Phase I/II BURGUNDY trial in nAMD (n=251, NCT04567303) is still recruiting patients. Study completion is set for 2027.
Sozinibercept/OPT-302 (Opthea)
Opthea last year adopted sozinibercept as the nonproprietary drug name of this agent that targets VEGF-C and D. Recruitment is continuing in Phase III trials in nAMD: ShORe (n=990, NCT04757610) and COAST (n=990, NCT04757636). They’re evaluating intravitreal OPT-302 2 mg in combination with ranibizumab 0.5 mg or aflibercept 2 mg, respectively. The primary endpoint for both studies is superiority in visual acuity gains at 12 months for the combination therapy vs. standard-of-care monotherapy.
In the DME program, 12-week results of the Phase Ib trial of sozinibercept/aflibercept combination therapy (n=9) demonstrated BCVA improvement of +7.7 letters and CST improvement of –71 mm. The Phase I/II trial (NCT03397264) enrolled 153 patients total. Opthea says it plans to advance the DME program. The company also reported progress in its manufacturing capabilities for sozinibercept.2
Tarcocimab tedromer/KSI-301 (Kodiak Sciences)
Tarcocimab is an anti-VEGF antibody biopolymer conjugate that’s being studied in two Phase III trials: GLOW2 in DR (n=250, NCT06270836); and DAYBREAK in nAMD (n=675, NCT-6556368). In the latter, tarcocimab is being evaluated alongside Kodiak stablemate tabirafusp tedromer, also known as KSI-501, which is an anti-IL-6, VEGF-trap bispecific antibody biopolymer conjugate designed to also inhibit VEGF-mediated angiogenesis and vascular permeability. In 2023, Kodiak announced it would discontinue the program after the GLEAM (n=460, NCT04611152) and GLIMMER (n=459, NCT04603937) trials in DME failed to demonstrate noninferiority vs. aflibercept, but Kodiak changed course when the GLOW Phase III trial in NPDR (n=253, NCT05066230) demonstrated a statistically significant improvement in vision compared to sham.
UBX1325/Fuselotoclax (Unity Biotechnology)
UBX1325 is a potent small-molecule inhibitor of B-cell lymphoma-extra-large (Bcl-xL) designed to inhibit the function of proteins that senescent cells rely on for survival. Unity extended the Phase IIb ASPIRE study (n=52, NCT06011798) in DME from 24 to 36 weeks and increased enrollment. The company says it expects to report 24-week data this year. Phase II studies evaluating safety, efficacy and tolerability in DME (n=65, NCT084857996) and AMD (n=51, NCT05275205) have been completed.
Umedaptanib pegol (Ribomic)
Umedaptanib pegol, formerly RBM-007, is an antifibroblast growth factor-2 aptamer that was the focus of four clinical trials in nAMD, all of which have been completed: Phase I SUSHI (n=9, NCT03633084), which demonstrated safety and tolerability; and Phase II TOFU (n=94, NCT04200248), RAMEN (n=40, NCT04640272) and TEMPURA (n=5, NCT04895293). TOFU evaluated intravitreal umedaptanib pegol monotherapy and combination therapy with aflibercept vs. aflibercept monotherapy. No study updates have been posted since 2023, when the company said it needed to evaluate umedaptanib pegol in early nAMD and alongside anti-VEGF agents. Ribomic had no updates the programs, but still lists the candidate on its website as active.
Xiflam/Tonabersat (InflammX)
Last year this candidate was listed as Tonabersat, formerly Xiflam, but Inflammx called it Xiflam in a press release early in 2025. It’s an oral, small-molecule NLRP3 inflammasome inhibitor that targets the Connexin43 protein and blocks the formation of hemichannels. The Phase II trial in DME (n=128, NCT05727891) completed enrollment but is still analyzing data.
REFERENCES
Mehta H, Wootton K, Simpson M, Mackey G, Naimark W. Efficacy and safety of the low dose dexamethasone IBE-814 IVT implant for diabetic macular edema and retinal vein occlusion: Reults of a first-in-human Phase 2 trial. Paper presented at Euretina 2024; Barcelona, Spain; September 22, 2024.
Boyer DS, Steinle NC, Pearlman JA, et al. Phase 1b dose escalation study of sozinibercept inhibition of vascular endothelial growth factors C and D with aflibercept for diabetic macular edema. Trans Vis Sci Tech. 2024;13:32.
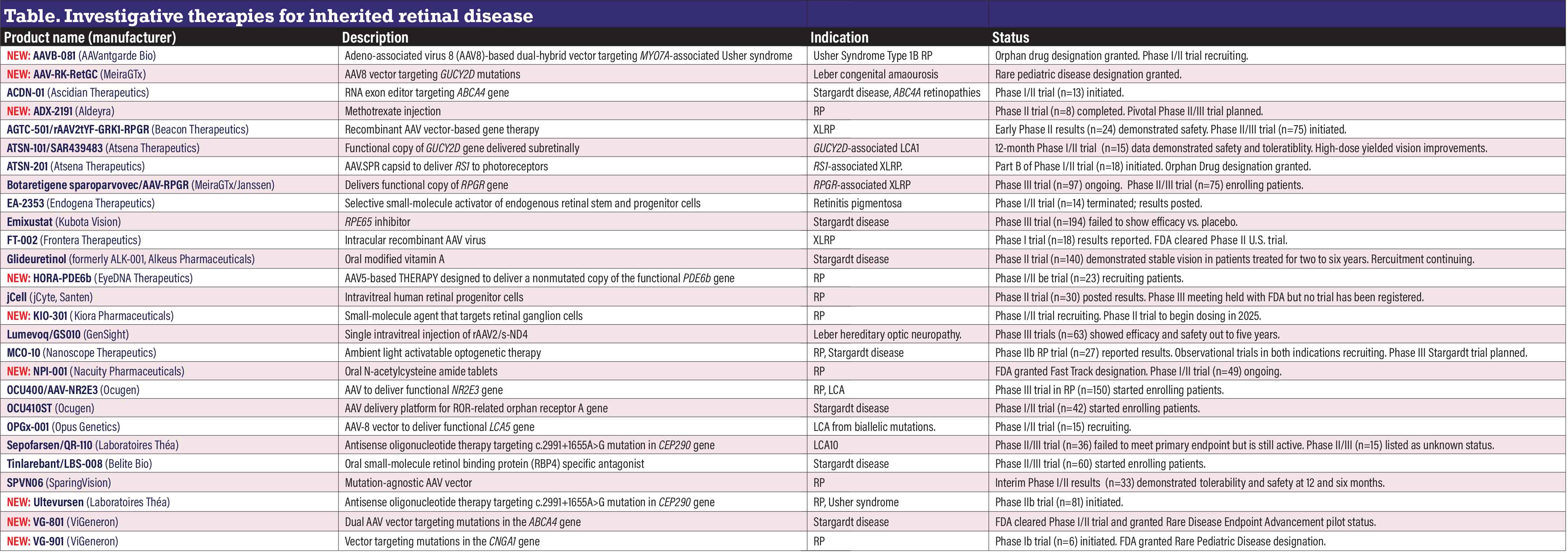
Investigative therapies for inherited retinal disease
Nine new candidates have been added, bringing the total count to 27. The following agents listed in 2024 have been removed from this year’s list:
- 4D-110 and 4D-125 (4D Molecular Therapeutics) are no longer listed on 4DMT’s website.
- FT-001 (Frontera Therapeutics). The ClinicalTrials.gov listing hasn’t been updated since 2023.
These candidates are carryovers from last year’s listing but either appear under a new name or with a name added in the past year:
- AGTC-501 (Beacon Therapeutics), now also laruparetigene zovaparvovec, or laru-zova.
- Lumevoq (GenSight Biologics), previously GS010 and also called lenadogene nolparvovec.
NEW: AAVB-081 (AAVantgarde Bio)
AAVB-081 is an intraretinal adeno-associated viral (AAV)-8-based dual hybrid product targeting MYO7A-associated Usher syndrome 1B retinitis pigmentosa. The Food and Drug Administration last year granted it Orphan Drug Designation. The dual-
hybrid platform uses two AAV8 vectors, each containing one half of an expression cassette encoding for the MYO7A gene and works at the cell nucleus level, recombining the two halves of the transgene back into one within the cell. The Phase I/II trial (n=15, NCT06591793) is recruiting.
NEW: AAV-RK-RetGC (MeiraGTx)
The FDA granted Rare Pediatric Disease Designation. The treatment is indicated for Leber congenital amaurosis due to GUCY2D mutations. No trial has been posted at ClinicalTrials.gov.
ACDN-01 (Ascidian Therapeutics)
The Phase I/II STELLAR trial (n=13, NCT06467344) started last year for this RNA exon editor delivered via a single vector targeting the ABCA4 gene in Stargardt disease and related retinopathies. The FDA granted Fast Track designation. An observational prescreening trial (n=50, NCT06445322) started recruiting in 2024.
 |
| Click here to view the table in the digital edition. |
NEW: ADX-2191 (Aldeyra)
ADX-2191is a methotrexate injection for RP due to rhodopsin misfolding mutations. The Phase II trial (n=8, NCT05392179) has been completed, and Aldeyra says it plans to initiate a Phase II/III trial. The Phase II trial demonstrated improvements in retinal sensitivity following treatment.
AGTC-501/rAAV2tYF-GRK1-RPGR (Beacon Therapeutics)
AGTC-501, now also carrying the label laruparetigene zovaparvovec, or laru-zova, is a recombinant AAV vector-based therapy targeting mutations in the RPGR gene. The Phase I/II HORIZON trial in X-linked RP (n=29, NCT03316560) is continuing recruitment. The Phase II/III VISTA trial in XLRP (n=75, NCTO4850118) started recruiting patients this year. Interim three-month results from the Phase II DAWN trial in XLRP (n=24, NCT06275620) demonstrated tolerability and no treatment-related adverse events, along with early gains in low-
luminance visual acuity.
ATSN-101/SAR439483 (Atsena Therapeutics)
ATSN-101, formerly known as SAR439483, is a subretinal AAV-based therapy for LCA caused by biallelic mutations in the GUCY2D gene. Twelve-month data from the Phase I/II trial (n=15, NCT03920007) demonstrated tolerability and improvement in visual function.1 Patients in the high-dose group (1x1011 vector genes [vg] per eye) had a 20.3-decibel improvement in full-field stimulus test and an eight-letter improvement in visual acuity.
ATSN-201 (Atsena Therapeutics)
Atsena initiated Part B of the Phase I/II LIGHTHOUSE trial in X-linked retinoschisis (n=21, NCT05878860). Part A of the study evaluated the safety and tolerability of three different doses administered subretinally. The data monitoring committee recommended proceeding to Part B using the 1x1011 vg/mL dose. The FDA granted Orphan Drug Designation for the indication. ATSN-201 uses the novel spreading capsid AAV.SPR to facilitate delivery of RS1 to photoreceptors in the fovea.
Botaretigene sparoparvovec/AAV5-RPGR (MeiraGTx/Janssen)
Through a one-time administration, botaretigene sparoparvovec (bota-vec) is designed to deliver functional copies of the RPGR gene that may counteract the loss of retinal cells in RPGR-associated XLRP. The Phase III LUMEOS trial (n=97, NCT04671433) completed enrollment while a new Phase II/III trial (n=75, NCT04850118) started.
EA-2353 (Endogena Therapeutics)
EA-2353 is a small molecule targeting progenitor cells in the ciliary body that promote retinal degeneration. The Phase I/IIa trial in RP (n=14, NCT05392751) was terminated last year. The trial enrolled four dose-escalating cohorts. The first three cohorts demonstrated improvements in BCVA and LLVA vs. untreated eyes.3 The company still lists the trial as active.
Emixustat (Kubota Vision)
This once-daily oral tablet aims to inhibit RPE protein 65 (RPE65) by blocking the accumulation of lipofuscin and vitamin A toxins. The Phase III trial in Stargardt disease (n=194, NCT03772665) was completed last year and demonstrated no clinically significant change in macular atrophy vs. placebo. Kubota still lists the program on its website.
FT-002 (Frontera Therapeutics)
This intraocular recombinant AAV vector targets XLRP. Preliminary data from the Phase I trial in China (n=18, NCT05874310) demonstrated safety and tolerability, with some dose groups having improvements in retinal sensitivity and visual function.4 The FDA cleared Phase II clinical trials in the United States. The therapy uses a recombinant AAV to deliver a codon-optimized gene encoding a GTPase modulator (hRPGRORF15) to retinal cells.
Glideuretinol/ALK-001 (Alkeus Pharmaceuticals)
Oral gildeuretinol acetate targets vitamin A to reduce its dimerization without modulating the visual cycle. Alkeus received FDA RPD and Fast Track designations, and previously received Breakthrough Therapy and Orphan Drug designations. The Phase II TEASE trial in Stargardt disease (n=140, NCT02402660) is recruiting patients. A Phase II open-label extension trial in Stargardt (n=140, NCT04239625) is also enrolling patients. Interim data from the TEASE-3 study demonstrated treated patients with early stage Stargardt had no disease progression and relatively stable vision, and stayed asymptomatic for up to six years.
NEW: HORA-PDE6b (eyeDNA Therapeutics)
HORA-PDE6b is an AAV5-based therapy designed to deliver a nonmutated copy of the functional PDE6b gene in the eyes of patients with RP caused by biallelic mutations of the target gene. The FDA granted RPD. The Phase I/II trial in RP (n=23, NCT03328130) is recruiting patients. HORA-PDE6b is placed in the subretinal space.
jCell (jCyte, Santen)
The Phase II trial in adults with RP (n=30, NCT04604899) has been completed. The trial demonstrated acceptable safety and tolerability with a signal of efficacy for BCVA and visual function. jCyte said it plans to start a Phase III trial in the second half of 2024, but no such trial has been registered at ClinicalTrials.gov. jCell is an intravitreal injection of human retinal progenitor cells indicated for RP.
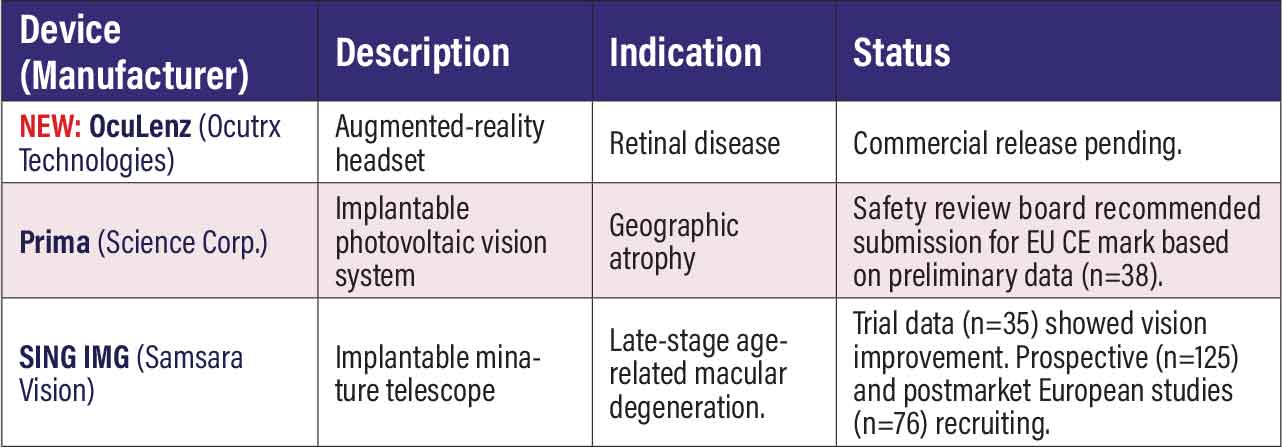
Investigative device-based therapies OcuLenz (Ocutrx Technologies). Ocutrx said it had anticipated releasing this headset for people with retinal disease in early 2024, but the company is still taking preorders on its website. The device uses augmented reality to overlay high-contrast, pixel-manipulated images onto the user’s remaining viable field of view. It has 2,500 resolution per eye and a field of view 60 x 40 x 72 degrees horizontally, vertically and diagonally. A Qualcomm Snapdragon XR2 processor powers the device, and it has Wi-Fi and cellular connectivity. Prima (Science Corp.). Science Corp. last spring acquired this technology from Pixium Vision. Prima is an intraocular implant consisting of a photovoltaic subretinal prosthesis for patients with vision loss from atrophic dry AMD. Based on preliminary clinical trial results from the European pivotal study (n=38, NCT04676854), the data safety monitoring board found the device was ready to move forward to receive the European Union CE mark. The study demonstrated that implant recipients had real-form vision restored and could read letter sequences with a clinically significant improvement of acuity. SING IMT (Samsara Vision). A readout of trial results from Italy (n=35) reported intermediate-term visual and safety outcomes of the implantable SING IMT (Smaller-Incision New-Generation Implantable Miniature Telescope) six months postsurgery.1 Researchers found that SING IMT implantation improved distance and near vision, with a low impact on the corneal endothelium cell density and manageable safety outcomes. The implant is a 10.8-mm telescope implanted in the eye through a small incision similar to cataract surgery. The prospective CONCERTO study (n=125, NCT05438732) is still recruiting older adults living with stable, bilateral central scotomas due to late-stage AMD and fovea-involving geographic atrophy or disciform scar. A postmarket study in Europe of late-stage AMD patients who received the implant (n=76, NCT04796545) is continuing to recruit patients.
REFERENCE Toro MD, Savastano A, Aroca FV, et al. Smaller-incision new-generation implantable miniature telescope in late-stage age-related macular degeneration: 6 month outcomes. Heliyon. 2025;11:e41116. |
NEW: KIO-301 (Kiora Pharmaceuticals)
The FDA cleared the Phase II ABACUS-2 trial in RP (n=36, NCT06628947), with dosing to begin this year. The Phase I/II dose-escalation trial (n=102, NCT05282953) is still recruiting. KIO-301 is a small molecule that targets retinal ganglion cells.
Lumevoq/lenadogene nolparvovec (GenSight Biologics)
Formerly known as GS010, lenadogene nolparvovec is a single intravitreal injection of rAAV2/2-ND4 for patients with Leber congenital optic neuropathy due to a mutated ND4 mitochondrial gene. Five-year results from two Phase III trials (n=37, NCT02652780; n=39, NCT02652767) showed sustained bilateral improvement in BCVA and acceptable safety.2
MCO-010 (Nanoscope Therapeutics)
Nanoscope is pursuing two indications for MCO-010: RP and Stargardt disease. The MCO platform is designed to deliver light-sensitive multicharacteristic opsins into retina cells. The Phase IIb RESTORE trial in RP (n=27, NCT04945772) demonstrated improvement in functional vision and BCVA vs. sham. Nanoscope says it’s planning a Phase III trial in Stargardt disease. Two observational, long-term follow-up studies started in 2023 are still enrolling by invitation only: RP (n=18, NCT06162585); and Stargardt (n=6, NCT048185).
NEW: NPI-001 (Nacuity Pharmaceuticals)
The FDA last year granted Fast Track designation to NPI-001, N-acetylcysteine amide tablets, for RP associated with Usher syndrome. The Phase I/II trial (n=49, NCT04355689) is ongoing.
OCU400/AAV-NR2E3 (Ocugen)
This modified gene therapy targets nuclear hormone receptors for RP associated with mutations in the Nr2e3 gene and rhodopsin, as well as LCA with mutations in the CEP290 gene. The Phase III liMeliGhT RP trial (n=150, NCT063882000) dosed its first patient. Phase I/II data (n=22, NCT05203939) showed 10-letter improvement in LLVA in the treated eye.
OCU410ST (Ocugen)
The Phase I/II trial in Stargardt disease (n=42, NCT05956626) is recruiting. OCU410 uses an AAV platform for the retinal delivery of the RORA (ROR-related orphan receptor A) gene. FDA Orphan Drug designation, granted in 2023, covers treatment of ABCA4-associated retinopathies, including Stargardt disease, RP 19 and cone-rod dystrophy 3.
OPGx-001/OPGx-LCA5 (Opus Genetics)
The FDA granted RPD designation for this ocular gene therapy to treat LCA. OPGx-001 is an AAV8 vector designed to precisely deliver a functional LCA5 gene to the outer retina. The Phase I/II trial (n=15, NCT05616793) is recruiting patients.
Sepofarsen/QR-110 (Laboratoires Théa)
Although the Phase II/III ILLUMINATE trial in LCA10 (n=36, NCT03913143) failed to meet its primary endpoint of improved BCVA, the program is still active. The pediatric dose-escalation trial (n=15, NCT04855045) is listed as unknown status. Sepofarsen is an RNA therapy for LCA10 due to the c.2991+1655A>G mutation in the CEP290 gene.
Tinlarebant/LBS-008 (Belite Bio)
The Phase II/III DRAGON II trial in Stargardt disease (n=60,NTC06388083) started enrolling patients last year. Tinlarebant is an oral therapy that aims to reduce the accumulation of vitamin A-based toxins that cause retinal disease.
SPVN06 (SparingVision)
One-year results from the low-dose cohort of the Phase I/II PRODYGY trial in RP (n=33, NCT05748873) showed a favorable safety profile, as did six-month safety results for the medium-dose. Enrollment and treatment administration of the higher dose cohort commenced last fall. SPVN06 is a mutation-agnostic, AAV gene therapy composed of one neurotrophic factor (rod-derived cone viability factor, RdCVF) and one oxidative stress-reducing enzyme (RdCVF long form), designed to act together to slow or stop photoreceptor degeneration.
NEW: Ultevursen (Laboratoires Théa)
Ultevursen is an intravitreal antisense oligonucleotide therapy indicated for RP or Usher syndrome that targets mutations in the USH2A gene. The first patient was dosed in the Phase IIb LUNA clinical trial (n=81, NCT06627179).
NEW: VG801 (ViGeneron)
The FDA has cleared the Phase I/II study of VG801, a dual AAV vector that targets mutations in the ABCA4 gene in Stargardt disease and associated retinal dystrophies. ViGeneron says it also plans to submit a clinical trial application to the European Medicines Agency. The trial isn’t yet listed at ClinicalTrials.gov. The FDA granted VG801 Rare Disease Endpoint Advancement pilot status.
NEW: VG901 (ViGeneron)
VG901 targets RP caused by mutations in the CNGA1 gene. The Phase Ib trial (n=6, NCT06291935) started recruiting patients last year, with dose-escalation planned. The FDA also granted RPD designation. RS
REFERENCES
Yang P, Pardon LP, Ho AC, et al. Safety and efficacy of ATSN-101 in patients with Leber congenital amaurosis caused by biallelic mutations in GUCY2D: A Phase 1/2, multicentre, open-label, unilateral dose escalation study. Lancet. 2024;404:962-970.
Yu-Wai-Man P, Newman NJ, Biousse V, et al for the LHON Study Group. Five-year outcomes of lenadogene nolarvovect gene therapy in Leber hereditary optic neuropathy. JAMA Ophthalmol. Published online ahead of print; December 19, 2024.
Lam BL. First-in-human study to evaluate safety, tolerability and exploratory efficacy of a photoreceptor regeneration treatment with EA-2353 in patients with retinitis pigmentosa. Paper presented at ninth annual Retinal Cell & Gene Therapy Innovation Summit 2024. May 3, 2024; Seattle, Washington.
Sui R. Preliminary safety and efficacy results from an open-label, dose-escalation pilot gene therapy study of FT-002 in subjects with RPGR gene mutation associated X-linked retinitis pigmentosa. Paper presented at ninth annual Retinal Cell & Gene Therapy Innovation Summit 2024. May 3, 2024; Seattle, Washington.