 |
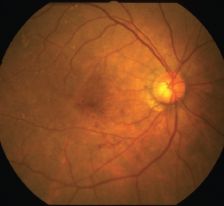
The ability to discern which patients with age-related macular degeneration are at higher risk of progressing to late AMD is a distinction that can make care more targeted and timely. OCT has contributed greatly to these efforts by allowing the detection and tracking of key biomarkers; however, the accuracy of these predictions has been called into question.
A new study published in Ophthalmic and Physiological Optics evaluated whether OCT biomarkers more accurately predict AMD progression compared to traditional color fundus photography, the results of which suggest that the complex interpretability of high-risk OCT biomarkers make it an ongoing challenge for practice integration.1
The retrospective study included 78 single eyes with intermediate AMD. Participants were an average of 71.8 years of age, 56 percent were women and 50 percent of white race/ethnicity. The groups were propensity-score matched by age and sex between converters and non-converters to late AMD.
Researchers used 10 specific OCT biomarkers and two fundus photo biomarkers (large drusen and pigmentary abnormality), which were independently graded by three researchers. Statistical analyses were then performed to evaluate biomarker prevalence, reliability of grading, time to conversion and their ability to predict late AMD.
According to the results, the adjusted risk was highest for OCT-detected nascent geographic atrophy, shallow irregular RPE elevations, drusenoid pigment epithelium detachment and RPE reflective abnormality (odds ratios, 6.66 to 28.27).
Fundus-detected pigmentary abnormalities demonstrated the highest individual prognostic accuracy (77.7 percent AUC), with excellent sensitivity (92.3 percent) but moderate specificity (63.1 percent). Adding at least three OCT biomarkers was required to improve prognostic performance significantly (91 percent), and at least eight additional biomarkers to yield both excellent sensitivity (94.6 percent) and specificity (90.8 percent).
They also note that, although biomarkers such as nascent GA and shallow irregular RPE elevation were strongly associated with progression to late AMD, their relatively lower prevalence likely limited their prognostic utility. “As such, high-risk but less common biomarkers may not be as useful in future imaging-based prognostic models unless supported by more common biomarkers” such as reticular pseudodrusen, they write. “Large drusen also lacked discriminatory power, though likely because they were nearly ubiquitous in eyes with intermediate AMD. Meanwhile, pigmentary abnormality emerged as the single most useful predictor of disease progression, combining moderate prevalence in both converter and non-converter eyes with strong discriminatory performance.”
The reliability of the grading personnel to identify biomarkers on OCT underscores the need for biomarkers that non-specialists can identify objectively and automatically, possibly with the use of artificial intelligence, the authors say.
Some weaknesses mentioned in the study include its relatively small sample size from a single eye-care center and the use of one type of OCT device, which limits how broadly the findings can be applied.
Additionally, internal validation through data splitting or cross-validation wasn’t performed due to the constraints of the sample size, possibly increasing the risk of overfitting during the biomarker selection.
“Finally,” continued the authors, “manual identification of biomarkers is time intensive and subject to inter-grader variability, although automated image interpretation is anticipated to become increasingly widespread.” These approaches will continue to require validation against expert consensus as the reference standard to ensure ongoing relevance, they noted.
Ultimately, the authors say this study confirms the reliability of fundus photography as a crucial tool in predicting AMD progression, and more common use of OCT biomarkers will require some form of automated identification in order to improve its efficiency in clinical settings.
REFERENCE
1. Trinh M, Cheung R, Nam J,Ng D, Nivison-Smith L, Ly A. High risk does notguarantee high accuracy—Evaluating theprognostic accuracy of OCT biomarkers forpredicting late AMD. Ophthalmic Physiol Opt. 2025;00:1–9. https://doi.org/10.1111/opo.13547
Blue-light Blocking May Not Affect AMD Incidence
Since the natural aging lens absorbs blue light, its replacement with a clear intraocular lens during cataract surgery has raised concerns that increased retinal exposure to blue light may make the retina and retinal pigment epithelium more vulnerable, potentially elevating the risk of age-related macular degeneration development. Evidence supporting this has been scant, however.
As such, researchers in South Korea sought to determine whether blue-light filtering IOLs effectively reduce AMD incidence in everyday environments. Their study, published in the American Journal of Ophthalmology, assessed the risk of wet AMD in patients who underwent bilateral blue light-filtering IOL implantation within one year compared to that of those with clear IOLs.1 They found that blue light-filtering IOL implantation in cataract surgery doesn’t reduce the risk of developing wet AMD in a South Korean population, regardless of a history of dry AMD.
The nationwide, population-based cohort study used data from the Korean National Health Insurance Sharing Service database. A total of 21,741 individuals with blue-light filtering IOLs were compared to 56,357 individuals with clear IOLs. The mean follow-up duration was seven years in the blue light-filtering IOL group and 6.7 years in the clear IOL group.
The 10-year cumulative incidence of wet AMD was 1.8 percent in the blue-blocking IOL group and 1.7 percent in the clear IOL group. The incidence rates per 100,000 person-years were 162.8 and 153.9 in the blue light-filtering IOL group and clear IOL group, respectively, with no significant difference using two statistical models: the adjusted Cox model (adjusted hazard ratio; aHR: 1.12) and the Fine-Gray model (aHR: 1.15). Subgroup analyses based on age, sex and history of dry AMD showed no protective effect of blue light-filtering IOLs, with comparable incidence rates across all subgroups.
“Despite substantial evidence from in vitro and animal model studies linking blue light-filtering IOLs and AMD, these lenses may fail to demonstrate effectiveness in clinical human studies because blue light exposure in daily life is not a major contributing factor to AMD,” the study authors write in their paper.
They wonder whether racial characteristics might play a role in AMD risk. South Korea is a predominantly single-ethnicity nation with a universal health care and social insurance system. East Asians generally have a higher concentration of melanin pigment in the iris and RPE, which may provide some protection against light-induced retinal damage.
Nevertheless, their findings further supported existing evidence that blue-blocking IOLs don’t provide a clear protective advantage against wet AMD. The research team called for further research to explore multiethnic populations, longer follow-up periods and other potential benefits of blue light-filtering IOL use. RS
REFERENCE
1. Kim JY, Kim S, Cho J, et al. Exudative AMD risk following blue light-filtering IOL implantation: A population-based study according to nonexudative AMD status. Am J Ophthalmol. July 11, 2025. [Epub ahead of print].
In Brief Ocugen announced the FDA cleared the Investigational New Drug amendment to initiate a Phase II/III pivotal confirmatory trial of OCU410ST, a modifier gene therapy candidate being developed for all Stargardt disease (ABCA4-associated retinopathies). Perfuse Shares Trial Results Perfuse Therapeutics announced results from two Phase II clinical trials of PER-001, a novel therapeutic designed to treat glaucoma and diabetic retinopathy. “Significant” improvement in vision was demonstrated compared to control in each trial, the company says. Topcon Launches IDHea Platform Topcon Healthcare announced the launch of the Institute of Digital Health (IDHea), an ocular data-as-a-service platform designed to accelerate AI research and digital health innovation by providing fast and secure access to real-world and clinical trial datasets. The company also acquired RetInSight, a Vienna, Austria-based developer of retinal imaging AI solutions, to advance its Healthcare from the Eye initiative. NPDR Trial Completes Phase Ib Breye Therapeutics announced the completion of its Phase Ib clinical trial evaluating its lead candidate, danegaptide, in patients with non-proliferative diabetic retinopathy with associated edema. Cognition Terminates Trial Cognition recently reported topline results from its Phase II COG2201 MAGNIFY trial of zervimesine (CT1812) in adults with GA secondary to dry AMD. The results show zervimesine-treated participants had 28.6 percent slower GA lesion growth on average and at 18 months, their lesions were 28.2 percent smaller compared to placebo, the company says. However, citing a business decision, the company has terminated the trial so it can focus on other indications. |



