Take-home points
|
 |
Bios Dr. Antaki is a vitreoretinal surgery fellow at the Cleveland Clinic Cole Eye Institute. Dr. Rachitskaya is a vitreoretinal surgeon at the Cleveland Clinic Cole Eye Institute. DISCLOSURES: Drs. Antaki and Rachitskaya have no financial interests to disclose. |
Despite having several things going for it pneumatic retinopexy continues to be underused in the United States due to how surgeons are trained and their preferences for procedures such as vitrectomy. However, PnR’s strong outcomes data and the convenience of being an in-office procedure make it a worthwhile option that surgeons shouldn’t ignore for the treatment of retinal detachment. Here, we’ll take a look at PnR’s pros and cons, and how it can be beneficial in selected patients.
Pneumatic retinopexy’s development
The evolution of PnR for retinal detachment repair stems from numerous innovations dating back to the early 20th century,1 culminating in its first formal introduction by Alfredo Domínguez of Madrid, Spain, in 1985.2 The technique was later coined and popularized by George F. Hilton and W. Sanderson Grizzard, former professors at the University of California Medical Center in San Francisco, in 1986.3 Early evidence emerged in 1989 when San Diego’s Paul Tornambe, MD, reported a single-surgery success rate of 73 percent for PnR in patients with detachments associated with breaks in the superior two-thirds of the retina.4 However, the modern resurgence of PnR is largely driven by a growing body of evidence-based literature comparing it to other surgical approaches. For example, the landmark PIVOT trial demonstrated an 80.8 percent initial success rate for PnR, along with superior visual acuity and less vertical metamorphopsia, but lower primary anatomic success compared to pars plana vitrectomy.5
There’s a growing interest in medicine toward office-based and outpatient treatments aimed at reducing hospital utilization and overall costs. PnR exemplifies this shift, offering an alternative that eliminates the need for anesthesia and an operating room. However, careful patient selection is essential to achieving optimal outcomes. In general, ideal candidates are phakic patients with an RRD involving a single break (or a few breaks spanning no more than 1 clock hour) between the 8 and 4 o’clock meridians. It’s recommended to avoid cases with inferior retinal pathology. That said, the PIVOT criteria included patients with breaks or lattice degeneration anywhere in the attached retina, as well as pseudophakic patients.5 Once PnR is chosen for RRD repair, several technical factors can influence the procedure’s success. These include the choice of gas tamponade, the use of cryotherapy versus laser retinopexy and the patient’s ability to maintain appropriate positioning.
Real-world results
While randomized controlled trial data suggest a high success rate for PnR, real-world data indicate lower success rates in practice. For example, an analysis of IRIS Registry data (2013 to 2022) reported a single-surgery success rate of only 59.82 percent for PnR overall, with pseudophakic eyes having an even lower success rate.6 A key limitation of such large-database data is the inability to account for clinical criteria used to determine whether a patient with RRD is an appropriate candidate for PnR. Moreover, in the study, operation failure was defined as the need for additional procedures, like repeat PnR, scleral buckle, PPV or complex retinal detachment repair. This may lead to an underestimation of the true success rate of PnR. For instance, in the early postoperative period, some surgeons may opt for further intervention to flatten the retina in the presence of persistent subretinal fluid, even though the retina might have reattached with observation without additional procedures in select cases. PnR is a non-drainage procedure that relies on the retinal pigment epithelium to pump out most of the fluid. Therefore, generally, if the break is sealed and no new breaks are present, residual fluid could sometimes be observed—as is common in SB surgery—and the case shouldn’t be considered a failure as long as the fluid resolves.
Even when PnR is considered successful, its durability may be questioned, as the underlying vitreous traction causing the retinal breaks isn’t addressed—unlike with SB or PPV. This is a valid critique of the approach, given that relieving traction is generally considered a core principle in the management of RRD. In a post-hoc analysis of the PIVOT trial with over five years of follow-up, both PnR and PPV were associated with very low rates of long-term redetachment.7 The authors suggest that eliminating vitreous traction through PPV may not be necessary for long-term reattachment in most RRDs. They draw a parallel with lasered horseshoe tears, which rarely cause detachments despite persistent traction. Older studies on PnR also support the long-term durability of this surgery.8
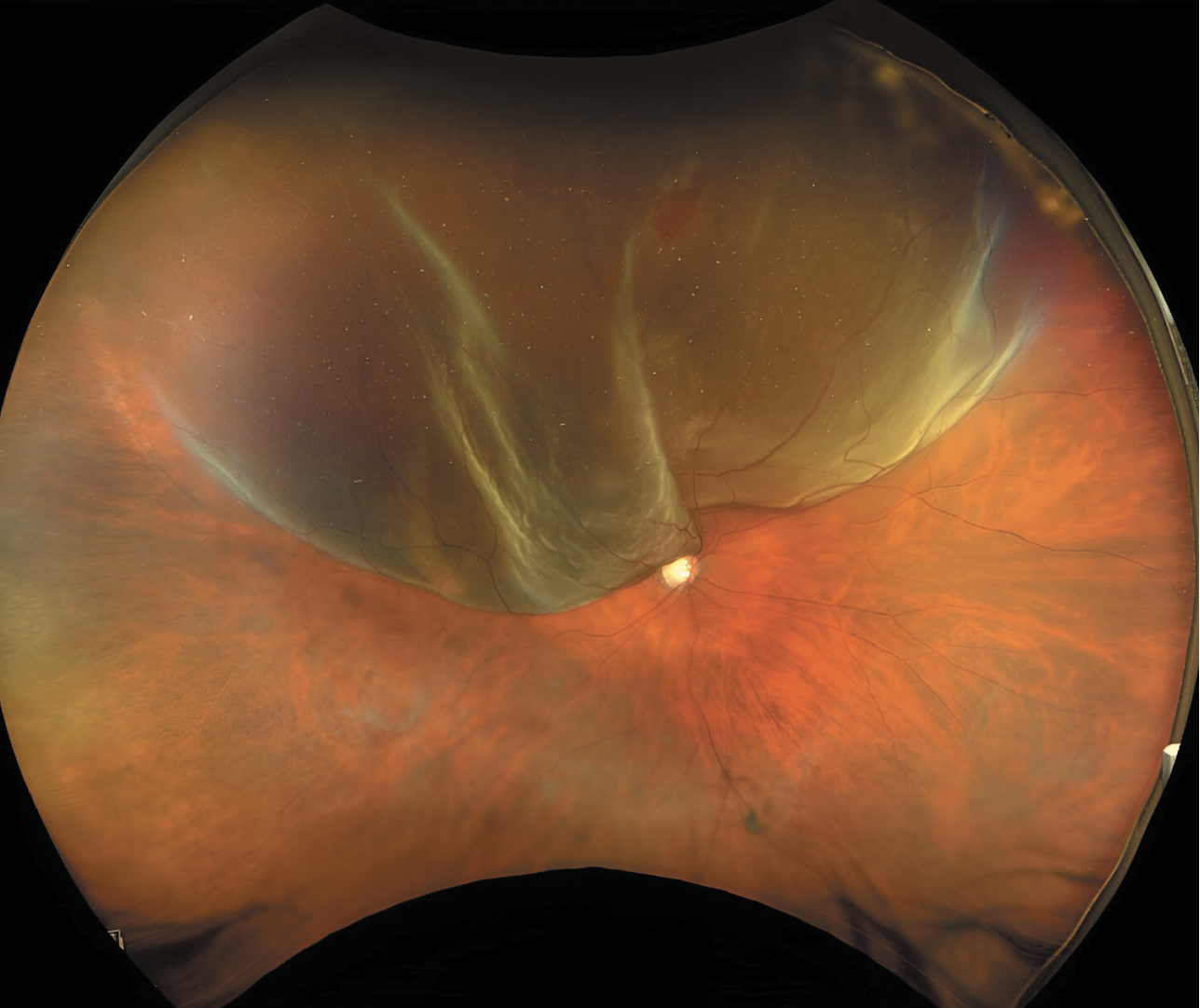 |
| Superior macula-splitting retinal detachment with a single horseshoe tear located between 12 and 1 o’clock in a phakic patient, an ideal candidate for pneumatic retinopexy. |
Current usage patterns
Overall, PnR appears to be underutilized in the United States, despite many detachments fitting the typical configuration for traditional PnR. For example, in 2021, only 36.1 percent of retina specialists responding to the ASRS Preferences and Trends Survey reported they would treat a phakic, superior, macula-on RRD with a single superior tear using PnR, and 58.1 percent said they performed PnR less than once per month.9 Its use varies significantly depending on the region, operating room availability and, to a large extent, surgeon comfort. Because PnR isn’t routinely taught in U.S.-based fellowships10—and given the greater familiarity with SB and PPV—surgeons tend to favor procedures they’re more comfortable performing. A laddered approach may be reasonable—starting with PnR and proceeding to PPV or combined SB/PPV if PnR fails. This strategy is supported by clinical trial evidence indicating that attempting PnR doesn’t compromise final reattachment rates or visual outcomes.4,5,11 Some evidence suggests, however, that the single-surgery anatomic success rate of PPV or SB following failed PnR may be lower—around 75 percent,12 compared to the higher success rates of 80 to 90 percent typically seen when PPV and/or SB are used as primary interventions for RRD.13,14
There is compelling evidence to use PnR as a first-line treatment for RRD because it’s been associated with better vision-related functioning (including higher mental health scores and improved ability to carry out daily activities) and less metamorphopsia compared to PPV.15,16 While those factors aren’t routinely measured in the clinic, from a practical standpoint, we also need to consider the risk of cataract progression.
In the PIVOT trial, 65 percent of phakic patients in the PPV arm underwent cataract surgery within one year, compared to 16 percent in the PnR group.5 This is a key practical consideration we routinely face after performing PPV or SB/PPV for RRD in phakic patients. The majority will require cataract surgery in the operated eye.
When planning subsequent cataract surgery, because these patients are often myopic in the fellow eye, intraocular lens calculations must take into account the risk of symptomatic aniseikonia. There are two main approaches: performing bilateral cataract surgery with refractive targets closer to plano; or operating only on the eye that developed a post-PPV cataract, targeting a moderate or high myopic outcome to match the fellow eye. The latter may represent a missed opportunity to improve the patient’s quality of life by achieving spectacle independence for distance vision.
The Cost Question
Regarding cost-effectiveness, it’s intuitive to assume that PnR, as an office-based procedure, is less expensive than PPV or SB, which require an operating room. The lower overhead and administrative costs in outpatient settings likely drive the perception of PnR as a more economical option.17 This may be significant for societies with a single-payer health-care system, where substantial savings could be realized when PnR is clinically appropriate. However, any economic analysis must also consider a broader range of factors beyond the initial procedure, such as follow-up visits, management of complications and the necessity for additional treatments. A recent economic analysis actually found that PPV was the most cost-effective primary procedure for primary RRD from the health-care payer perspective over a lifetime.18 This was attributed to PPV’s higher single-operation success rate, which helps avoid the additional costs associated with treatment failures.
AI’s potential impact
Recent advances in artificial intelligence have sparked early interest in applying these techniques to predict outcomes in RRD.19,20
For instance, one study explored the use of preoperative patient factors to predict the success of PnR.19 As large datasets of RRD cases become increasingly available—ideally enriched with ultra-widefield imaging and detailed clinical variables such as break location, extent of detachment and patient age—there is potential to build robust AI-based predictive models. We speculate that, eventually, by training models on thousands of RRD cases with known outcomes, AI could provide individualized predictions.
For example, a model could analyze a fundus image along with patient-specific factors to estimate the likelihood of success with PnR. It might even do better than the usual decision-making shortcuts doctors use by picking up on subtle features we might miss. Of course, we recognize that even with comprehensive datasets, numerous nuanced variables such as surgeon skill and patient compliance remain difficult to quantify. Still, the emergence of multimodal models that incorporate imaging, videos and even data from wearable sensors (e.g., tracking postoperative positioning) may bring us closer to personalized, AI-driven retinal care. Foundation models may play a key role in enabling this next frontier.21
Bottom line
In conclusion, PnR represents a compelling, safe, effective, first-line option for selected cases of RRD. Despite variability in real-world success rates and potential underutilization due to training gaps and surgeon preference, evidence from clinical trials supports its efficacy and long-term durability. With the rise of AI-driven predictive tools in medicine and growing interest in outpatient retinal care, PnR may play an increasingly important role in the future of personalized RRD management. RS
REFERENCES
1. Fernández-Vega González A, Muni RH. The history of pneumatic retinopexy: Have we come full circle? Acta Ophthalmol. 2022;100:1:118-120.
2. Domínguez A. Cirugía precoz y ambulatoria del desprendimiento de retina. Arch Soc Esp Oftalmo. 1985;48:47-54.
3. Hilton GF, Grizzard WS. Pneumatic retinopexy. A two-step outpatient operation without conjunctival incision. Ophthalmology. 1986;93:5:626-641.
4. Tornambe PE, Hilton GF. Pneumatic retinopexy. A multicenter randomized controlled clinical trial comparing pneumatic retinopexy with scleral buckling. The Retinal Detachment Study Group. Ophthalmology. 1989;96:6:772-783; discussion 784.
5. Hillier RJ, Felfeli T, Berger AR, et al. The Pneumatic Retinopexy Versus Vitrectomy for the Management of Primary Rhegmatogenous Retinal Detachment Outcomes Randomized Trial (PIVOT). Ophthalmology. 2019;126:4:531-539.
6. Griffin S, Chan L, McCarthy K, et al. Pneumatic retinopexy for rhegmatogenous retinal detachment outcomes: IRIS Registry (Intelligent Research in Sight) analysis. Ophthalmol Retina. 2025;9:5:437-443.
7. Chen TS, Motekalem Y, Melo IM, et al. Long-term redetachment rates of pneumatic retinopexy versus pars plana vitrectomy in retinal detachment: A PIVOT post hoc analysis. Ophthalmol Retina. 2024;9:2:122-126.
8. Eter N, Böker T, Spitznas M. Long-term results of pneumatic retinopexy. Arbeitsphysiologie. 2000;238:8:677-681.
9. Paul Hahn for The American Society of Retina Specialists. 2021 Membership Survey Preferences and Trends. Presented at: 2021. Accessed April 13, 2025. https://www.asrs.org/content/documents/asrs-2021-pat-survey-for-website.pdf.
10. Emami-Naeini P, Deaner J, Ali F, et al. Pneumatic retinopexy experience and outcomes of vitreoretinal fellows in the United States: A multicenter study. Ophthalmol Retina. 2019;3:2:140-145.
11. Ambler JS, Meyers SM, Zegarra H, Paranandi L. Reoperations and visual results after failed pneumatic retinopexy. Ophthalmology. 1990;97:6:786-790.
12. Anaya JA, Shah CP, Heier JS, Morley MG. Outcomes after failed pneumatic retinopexy for retinal detachment. Ophthalmology. 2016;123:5:1137-1142.
13. Joseph DP, Ryan EH, Ryan CM, et al. Primary retinal detachment outcomes study: Pseudophakic retinal detachment outcomes: Primary retinal detachment outcomes study report number 3. Ophthalmology. 2020;127:11:1507-1514.
14. Ryan EH, Ryan CM, Forbes NJ, et al. Primary retinal detachment outcomes study report number 2: Phakic retinal detachment outcomes. Ophthalmology. 2020;127:8:1077-1085.
15. Muni RH, Francisconi CLM, Felfeli T, et al. Vision-related functioning in patients undergoing pneumatic retinopexy vs vitrectomy for primary rhegmatogenous retinal detachment: A post hoc exploratory analysis of the PIVOT randomized clinical trial: A post hoc exploratory analysis of the PIVOT randomized clinical trial. JAMA Ophthalmol. 2020;138:8:826-833.
16. Brosh K, Francisconi CLM, Qian J, et al. Retinal displacement following pneumatic retinopexy vs pars plana vitrectomy for rhegmatogenous retinal detachment. JAMA Ophthalmol. 2020;138:6:652-659.
17. Elhusseiny AM, Yannuzzi NA, Smiddy WE. Cost analysis of pneumatic retinopexy versus pars plana vitrectomy for rhegmatogenous retinal detachment. Ophthalmol Retina. 2019;3:11:956-961.
18. Felfeli T, Teja B, Miranda RN, et al. Cost-utility of rhegmatogenous retinal detachment repair with pars plana vitrectomy, scleral buckle, and pneumatic retinopexy: A microsimulation model. Am J Ophthalmol. 2023;255:141-154.
19. Nisanova A, Yavary A, Deaner J, et al. Performance of automated machine learning in predicting outcomes of pneumatic retinopexy. Ophthalmol Sci. 2024;4:5:100470.
20. Antaki F, Kahwati G, Sebag J, et al. Predictive modeling of proliferative vitreoretinopathy using automated machine learning by ophthalmologists without coding experience. Sci Rep. 2020;10:1:19528.
21. Chia MA, Antaki F, Zhou Y, Turner AW, Lee AY, Keane PA. Foundation models in ophthalmology. Br J Ophthalmol. 2024;108:10:1341-1348.



