Take-home points
|
 |
Bios Dr. Ozdek is a professor of Dr. Ozdemir Zeydanli is a vitreoretinal surgeon at Ankara City Hospital. DISCLOSURES: The authors have no relevant financial disclosures. |
Pediatric retinal detachments aren’t just small adult RDs. They differ fundamentally from adult cases—not only in anatomy and etiology but also in timing, presentation and prognosis.1,2 Delayed diagnosis is common, with macular involvement or advanced proliferative vitreoretinopathy at presentation. Early, tailored intervention is critical due to amblyopia risk and the unique tissue behavior of the pediatric eye, such as tenacious vitreoretinal adhesion and robust healing response that often leads to rapid scarring.
Pediatric cases often have different goals—such as halting progression, making the anatomy less abnormal or simply preserving the globe or maintaining light perception. Here, “less is more” becomes a guiding principle: Aggressive maneuvers that may be tolerated in adults can cause inoperable damage in children when they result in iatrogenic retinal breaks.
This article offers practical tips, surgical strategies and key considerations for managing the different forms of pediatric RD—rhegmatogenous, tractional and exudative—with a focus on avoiding complications and optimizing outcomes.
Making the diagnosis
The first step in pediatric RD management is determining the type of detachment—rhegmatogenous, tractional or exudative—as this distinction guides surgical strategy and prognosis. A thorough history is essential and should include birth history, systemic or genetic conditions, prior trauma, high myopia and family history of RD.
A low threshold for examination under anesthesia is essential, especially when cooperation is limited. Retinoscopy, B-scan ultrasonography, widefield imaging and optical coherence tomography, when feasible, help define the extent and nature of the detachment (Figure 1). Always examine the fellow eye too, as subtle bilateral findings can suggest hereditary conditions or systemic diseases. If a hereditary condition such as Stickler, familial exudative vitreoretinopathy or X-linked retinoschisis is suspected, examining parents and siblings may reveal diagnostic clues.3
Critically, in all cases of unexplained leukocoria or RD, ruling out retinoblastoma is essential before proceeding with intervention.
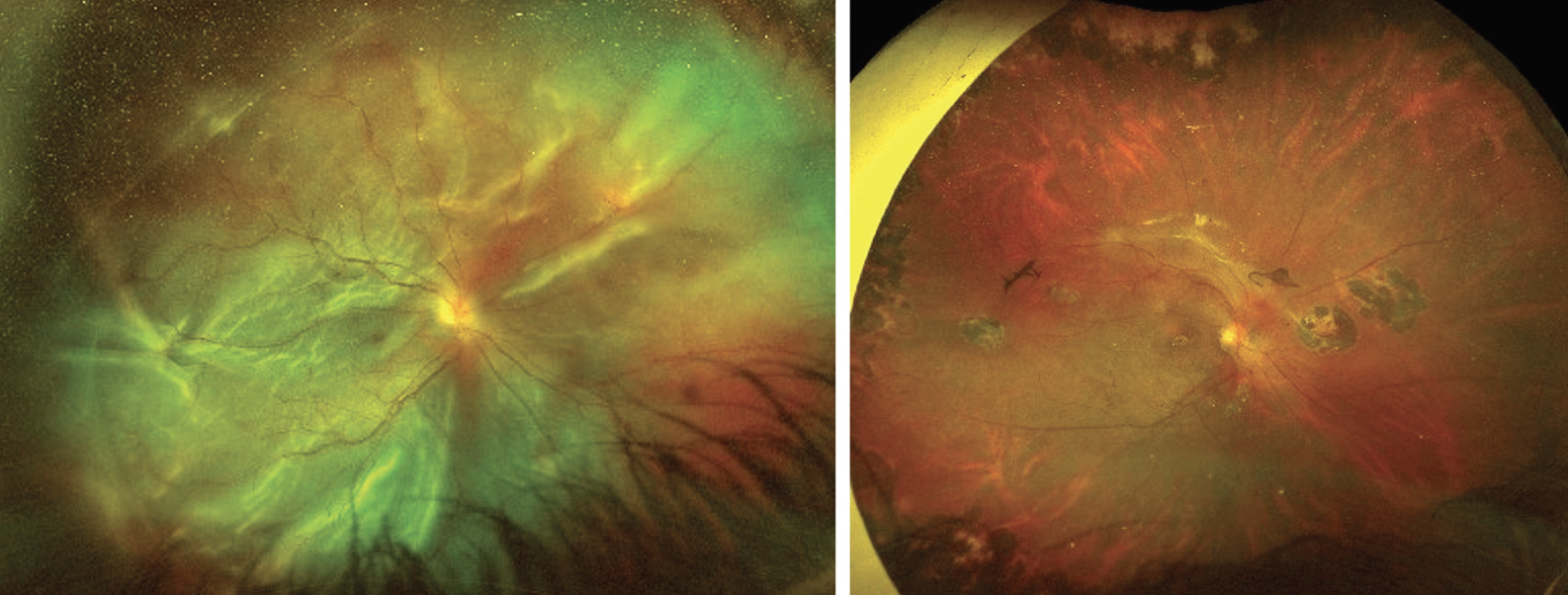
 |
| Figure 1. An infant with Knobloch syndrome, associated with COL18A1 mutation, presenting with shallow RD in a myopic fundus without apparent retinal break on examination. OCT reveals macular hole-related RD, which becomes apparent with the vitreous suction during surgery (arrow). |
Rhegmatogenous RD
Rhegmatogenous retinal detachments in children often have different mechanisms than in adults.1,2 Trauma is the most common cause and should always be suspected.4 When history and clinical findings don’t align, non-accidental trauma must be considered and reported, if necessary. In spontaneous RRDs, consider hereditary vitreoretinal dystrophies (e.g., Stickler, Marfan, X-linked retinoschisis) and prematurity.5,6 In such cases, prophylactic treatment of the fellow eye (e.g., peripheral laser or scleral buckle) may be warranted. Retinoscopy, which is crucial for the diagnosis, is usually overlooked, especially in babies.
High myopia should alert the physician for Stickler, Marfan, prematurity and
Knobloch syndrome, all of which may be associated with RRD. All children with RRD should be asked about the prematurity history specifically, since it’s usually forgotten by the parents when the child is grown up. Also, presence of encephalocele or skin defect at the back of the scalp should be checked for Knobloch syndrome.7 Buphthalmos is another important cause of RRD at pediatric age both because of thinned retina secondary to an enlarged globe and previous surgeries for glaucoma triggering posterior vitreous detachment. Children operated on for congenital cataract should also be followed closely for development of RRD.8
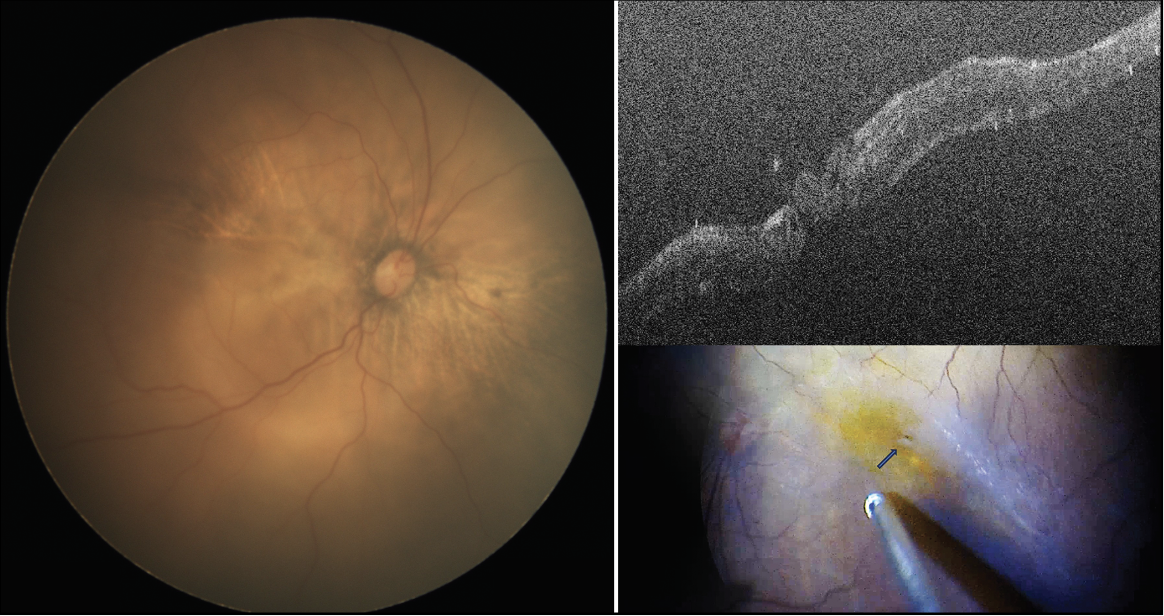
|
| Figure 2. A total rhegmatogenous retinal detachment in a 14-year-old male with a history of penetrating trauma at age 2 who presented with superior retinal dialysis, multiple retinal tears in all quadrants and associated proliferative vitreoretinopathy (left). Surgical management included membrane removal and adjunctive mitomycin-C application to prevent recurrence. The retina remained attached with no evidence of recurrent proliferation at two-year follow-up (right). |
• Surgical considerations. Scleral buckling remains the first-line treatment for most pediatric RRDs, given the challenges of separating the strongly adherent vitreous in these young eyes. Additional advantages include eliminating the need for postoperative positioning, lower risk of endophthalmitis and maintaining efficacy even in chronic inferior detachments with subretinal bands. Segmental or encircling buckles may be used depending on break location and extent. Special considerations for using SB in children include:
• In children under 4 years old or with delayed ocular growth, the band may need to be cut or removed after six months to allow normal growth of the eye. Since capsular tissue can form rapidly around the SB in pediatric eyes, division is preferred over removal to preserve support and minimize trauma.9
• Refractive shifts from SB can cause anisometropic amblyopia and should be closely monitored and corrected.
• Cryopexy is preferred intraoperatively as laser may not be feasible postop in uncooperative children.
• Subretinal fluid drainage is usually performed but may be omitted for shallow detachments.
• When to do vitrectomy in RRD? Vitrectomy is considered in eyes with vitreous hemorrhage, significant traction, total RD with multiple tears, giant retinal break, advanced PVR, pupillary dilatation problems, unseen retinal break(s), macular hole or buphthalmia with previous glaucoma surgeries where you need to preserve the conjunctiva from scarring.
In most of these cases, SB serves as an adjunct to vitrectomy by 1) supporting the vitreous base and helping lens preservation—especially when a complete posterior vitreous detachment can’t be achieved and 2) relieving part of the tractional forces secondary to PVR. When combined with vitrectomy, SB placement is usually a circumferential encircling band to provide full broad support anterior to the equator. Special considerations for performing vitrectomy in pediatric RRD include:
Sclerotomy placement must be age-adjusted,10 since the pars plana isn’t fully developed before 8 to 9 months after birth. Trocar cannulas should be placed perpendicular to the sclera to avoid trocar dislocation and lens damage.
Use lens-sparing techniques whenever possible. The insertion of intraocular instruments should always be parallel to the visual axis to spare the lens. Lens volume is larger in proportion to the volume of the whole globe as compared to the adult eye. Higher caution is necessary to avoid damage to the lens.
Inducing a posterior vitreous detachment is often difficult. If the usual induction of PVD with suction and cutter isn’t effective, forceps can be used to initiate hyaloid separation with the goal of extending it past the equator. When using forceps, one should intend to make a pinch from the internal limiting membrane to guarantee engaging the hyaloid under very high magnification. Otherwise, it would be difficult to catch the loose hyaloid directly.
Repeat triamcinolone staining is valuable to see the dissection planes. A PFCL bubble can stabilize the posterior pole and, once you start the PVD, it assists in propagating the PVD by entering in between the hyaloid membrane and the retina, helping dissection. Sometimes total vitreous removal may not be possible without the risk of breaks, and some parts of the vitreous may need to be left behind. In those conditions, one should always make sure that vitreous is totally removed at the margins of the break to prevent redetachment.
Silicone oil may be preferred over gas in young children due to less need for positioning, and lower amblyogenic/cataractogenic risk. Emulsification of SO occurs much earlier in children than adults, which is why higher viscosity SO such as 5,000 cs may be a good option. However, glaucoma risk is high—especially in myopic, oil-filled eyes—and requires close monitoring. Earlier removal as soon as two months may be considered in children to avoid such problems.
PVR is very frequent and severe in children, which necessitates extra precautions. Intravitreal methotrexate injections (both at the end of surgery and postoperatively)11 or intraoperative mitomycin-C application in a sandwich technique may be helpful to prevent PVR (Figure 2).12 Despite all these precautions, the anatomical and surgical success rates in children are lower than adults and the risk of multiple surgeries is high.2
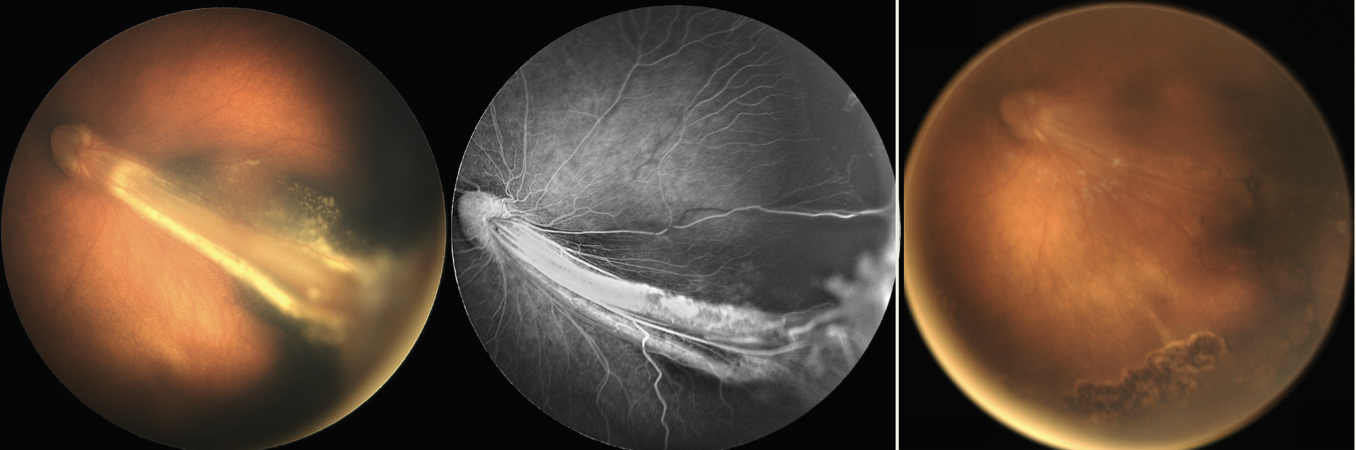 |
| Figure 3. Familial exudative vitreoretinopathy associated with macular fold, exudation and peripheral nonperfusion (left). Tractional retinal detachment resolved with flattening of macular fold two months after lens-sparing vitrectomy (right). |
Tractional RD
Pediatric TRDs are most common in the setting of retinopathy of prematurity, FEVR (Figure 3), and persistent fetal vasculature and, rarely, in incontinentia pigmenti and Norrie disease. Key principles in the management of pediatric TRDs include:
• Avoid creating retinal breaks. These eyes are often avascular with extensive fibrovascular proliferation, and an iatrogenic break can trigger relentless PVR. If a break does occur, a segmental scleral buckle may help reduce local traction and support the area.
• Staged surgery is often necessary. Complete retinal flattening at the end of the surgery is usually not possible in these cases. The primary goal is to safely release anterior-posterior and tangential tractions. Retinal flattening may continue to improve over time, and finer membranes that become apparent can eventually be addressed in a planned second surgery.
• Lens-sparing vitrectomy should be the preferred approach whenever possible. However, in advanced TRD with retrolental membranes and the retina-lens apposition, vitrectomy combined with lensectomy may be necessary.
• Anticipate potential anatomical variations. Before placing sclerotomies, the ora serrata–pars plicata region should be carefully examined for possible anomalies, such as anterior retinal elongations especially in anterior PFV cases or dragged retina as in FEVR and ROP. Limbal entry sites can be preferred until safe.13
• Peripheral avascular retina is part of the disease in most pediatric TRDs (ROP, FEVR, IP). Therefore, laser ablation of these areas is essential for long-term success. Also, asymmetrical bilateral disease is common. Always examine the fellow eye and perform fluorescein angiography when possible.
• Recognize anatomical limitations. In severe cases of PFV, Norrie disease or advanced stage 5 ROP, profound retinal dysplasia precludes meaningful visual recovery.14,15 These limitations should be discussed with the family early in the care process.
• Encourage genetic testing when possible. Genetic evaluation can aid in diagnosing atypical and challenging cases, identifying syndromic associations, guiding family counseling and facilitating early detection and treatment in siblings or future offspring.
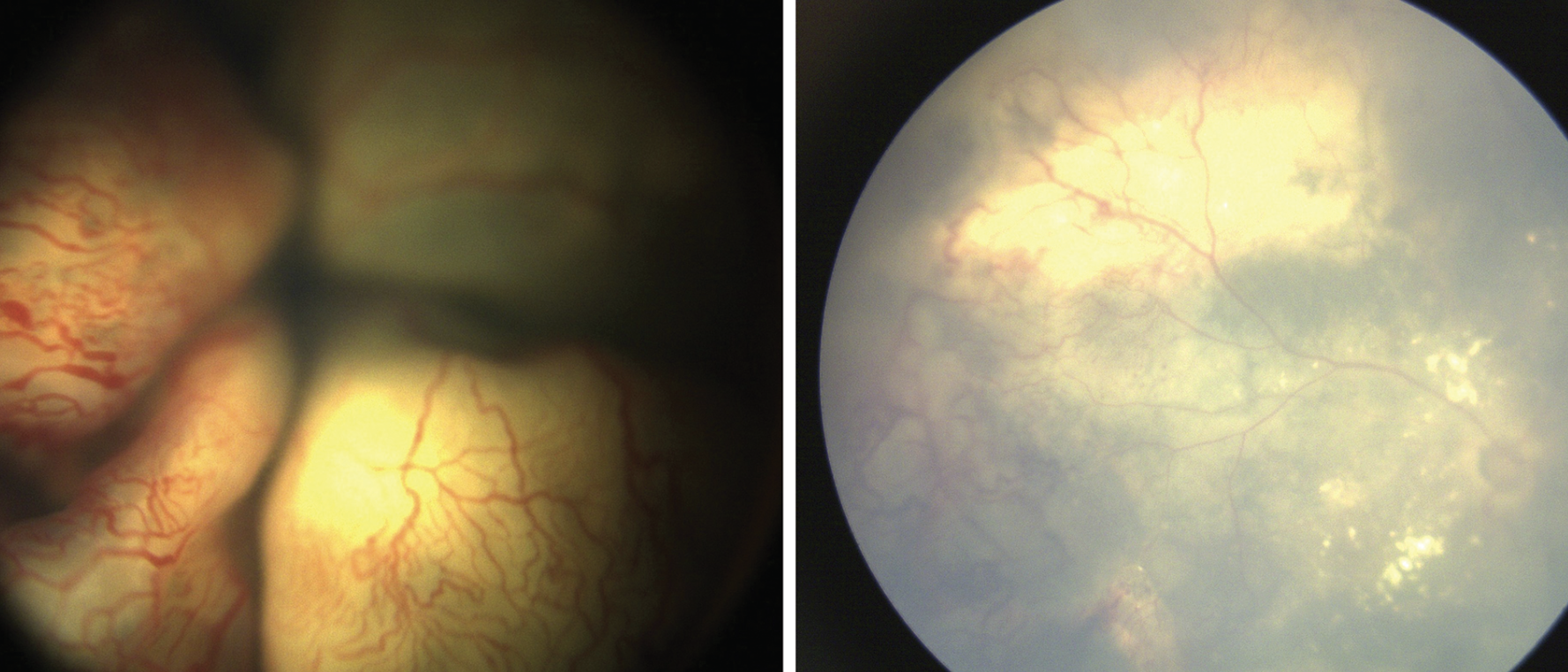 |
| Figure 4. Coats’ disease showing bullous exudative retinal detachment with engorged vessels and telangiectasias in an 18-month-old female (left). Ultrasound showed no mass lesion or calcification. Surgery with external drainage combined with vitrectomy, endolaser and cryotherapy, and anti-VEGF injection resulted in retinal reattachment (right). |
Exudative RD
Exudative RD in children is relatively uncommon and associated with underlying vascular, inflammatory, systemic conditions or malignancy. The most common pediatric causes include Coats’ disease, retinoblastoma, retinal hemangioblastoma, nanophthalmos and choroidal hemangioma. The primary management is to identify and treat the underlying cause and address the source of the exudation.
 |
Coats’ disease-related exudative RD may be a very representative entity considering the treatment plan. In early stage 3A Coats’ disease where there’s only a shallow partial RD, ablative treatments like laser photocoagulation and cryotherapy may be enough. The addition of intravitreal anti-vascular endothelial growth factor injections is usually helpful to control the disease. Lasers should be applied directly over all the dilated pathologic telangiectatic vessels as well as ischemic areas. However, when the retina is totally detached or highly elevated as in stage 3B disease, surgical interventions such as external drainage with or without vitrectomy together with ablative treatments become mandatory (Figure 4). Our group has previously reported that the need for additional interventions is much less and anatomical and functional results are better when vitrectomy is done together with external drainage.16
Bottom line
Managing pediatric retinal detachments demands more than surgical expertise—it requires an understanding of the unique anatomical and developmental aspects of the pediatric patient. From the initial encounter to surgical interventions and postoperative care, every step must be guided by the child’s systemic condition, etiology of detachment, long-term visual potential and the risk of amblyopia.
In rhegmatogenous detachments, scleral buckling remains a key technique, while in tractional cases, cautious and often staged release of traction is critical. Exudative RDs on the other hand hinge on recognizing and controlling the underlying pathology. Across all types, the focus should be on doing no harm: Iatrogenic breaks, lens damage or overly aggressive maneuvers may compromise the already limited visual potential.
Adhering to these principles—alongside close collaboration with families—offers the best opportunity to preserve ocular structure, maintain visual function and ultimately improve the child’s quality of life. RS
REFERENCES
1. Bowe T, Adams OE, Yonekawa Y. Management of pediatric rhegmatogenous retinal detachment. Semin Ophthalmol. 2025;40:4:283-287.
2. Nuzzi R, Lavia C, Spinetta R. Paediatric retinal detachment: A review. Int J Ophthalmol. 2017;10:10:1592-1603.
3. Ozdek S, Tefon Aribas AB, Atalay HT. Peripheral and central retinal vascular changes in asymptomatic family members of patients with familial exudative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2023;261:12:3449-3456.
4. Hoyek S, Baldwin G, Patel NA. Pediatric Traumatic Retinal Detachments. In: Özdek S, Berrocal A, Spandau U, eds. Pediatric Vitreoretinal Surgery. Springer International Publishing. 2023;493-503.
5. Enriquez AB, Baumal CR. Genetic Diseases Causing RRD: Marfan, Stickler and Wagner Syndrome. In: Özdek Ş, Berrocal A, Spandau U, eds. Pediatric Vitreoretinal Surgery. Springer International Publishing. 2023;473-492.
6. Özdemir Zeydanlı E, Özdek Ş, Küçükbalcı T. Surgical outcomes of rhegmatogenous retinal detachment associated with regressed retinopathy of prematurity. Turk J Ophthalmol. 2024;54:4:223-227.
7. Ozdek S, Zeydanli EO, Kayhan G, et al. Ophthalmological and genetic profile in knobloch syndrome: Ocular and genetic features of Knobloch syndrome. Am J Ophthalmol. 2025;13:277:87-95.
8. De La Huerta I, Williams GA. Rhegmatogenous retinal detachment after pediatric cataract surgery. Ophthalmology. 2018;125:1:4-5.
9. Gan NY, Lam WC. Special considerations for pediatric vitreoretinal surgery. Taiwan J Ophthalmol. 2018;8:4:237-242.
10. Lemley CA, Han DP. An age-based method for planning sclerotomy placement during pediatric vitrectomy: A 12-year experience. Trans Am Ophthalmol Soc. 2007;105:86-91.
11. Benner JD, Dao D, Butler JW, Hamill KI. Intravitreal methotrexate for the treatment of proliferative vitreoretinopathy. BMJ Open Ophthalmol. 2019;4:1:e000293.
12. Gürelik İG, Özdemir HB, Köse BG, Acar AB. Effect of adjuvant mitomycin-C on recurrent rhegmatogenous retinal detachment with proliferative vitreoretinopathy managed by relaxing retinotomy and retinectomy. J Ophthalmol. 2025;19:9927416.
13. Ozdemir Zeydanli E, Ozdek S, Acar B, Ozdemir HB, Atalay HT. Severe anterior persistent fetal vasculature: The role of anterior retinal elongation on prognosis. Graefes Arch Clin Exp Ophthalmol. 2023;261:10:2795-2804.
14. Walsh MK, Drenser KA, Capone A, Trese MT. Early vitrectomy effective for bilateral combined anterior and posterior persistent fetal vasculature syndrome. Retina Phila Pa. 2010;30:4:S2-8.
15. Walsh MK, Drenser KA, Capone A, Trese MT. Early vitrectomy effective for Norrie disease. Arch Ophthalmol Chic Ill 1960. 2010;128:4:456-460.
16. Ucgul AY, Ozdek S, Ertop M, Atalay HT. External drainage alone versus external drainage with vitrectomy in advanced Coats disease. Am J Ophthalmol. 2021;222:6-14.