Take-home points
|
 |
Bios Dr. Prasad is an associate clinical professor of ophthalmology at the Stein Eye Institute, Geffen School of Medicine, UCLA. Dr. Hou is an assistant professor of ophthalmology at the Stein and Doheny Eye Institutes, Geffen School of Medicine, UCLA. Dr. Schwartz is executive director of the Retinal Science Foundation. |
Despite advances in detection and management of diabetic eye disease, tractional retinal detachment continues to complicate advanced proliferative diabetic retinopathy and can result in visual impairment and blindness. Characterized by fibrosis associated with retinal neovascularization, diabetic TRDs may be further complicated by retinal breaks resulting in combined rhegmatogenous-tractional retinal detachment. Appropriate management can often stabilize or restore vision. Peripheral TRDs can remain stable for extended periods of time and therefore may be observed particularly when the ischemic drive is treated with anti-VEGF pharmacotherapy and/or panretinal laser photocoagulation. Progressive or macula-involving TRDs, on the other hand, often require surgical intervention. The spectrum of surgical indications for diabetic TRD is broad and varies depending on many factors including the degree of macular involvement, extent of ischemia, rate of progression, patient compliance, fellow-eye status and medical comorbidities.
Once the decision for surgery has been made, achieving optimal results requires a nuanced assessment of the patient’s anatomy, underlying perfusion status and other ocular co-morbidities. Surgical outcomes depend both on best practices and technical proficiency. The goals of surgery typically include media clearance, relief of retinal traction and treatment of peripheral retinal ischemia. Modern vitreoretinal surgical platforms including small-gauge surgical instrumentation, advanced fluidics and multimodal visualization options may improve anatomic and functional outcomes for patients with diabetic TRD.1
While there are many surgical approaches to diabetic TRD, this brief review covers surgical perspectives and tips to consider when managing these patients.
Preoperative assessment
When a patient presents with a possible TRD, here are the steps to follow in order to get the full picture before settling on a treatment approach:
• Comprehensive evaluation. Patients with advanced PDR often have multiple medical co-morbidities that also are ideally addressed and optimized prior to surgical intervention. Communication with the patient’s family, primary care physician and/or endocrinologist is necessary to evaluate the patient’s systemic health and assess candidacy for surgery. Optimization of glycemic control, blood pressure, renal function, anemia and management of anticoagulants can all have positive effects on the patient’s surgical course and, importantly, can help to decrease risk for additional ophthalmic and systemic complications.2
“Surgery starts with the physical exam.” Before any vitreoretinal surgery is undertaken, a thorough ophthalmic examination with detailed documentation is essential. Best-corrected vision, intraocular pressure and afferent pupillary defect status are critical prognostic factors to assess. Undilated examination for angle and iris neovascularization as well as assessment of corneal clarity and lens status are necessary for surgical planning.
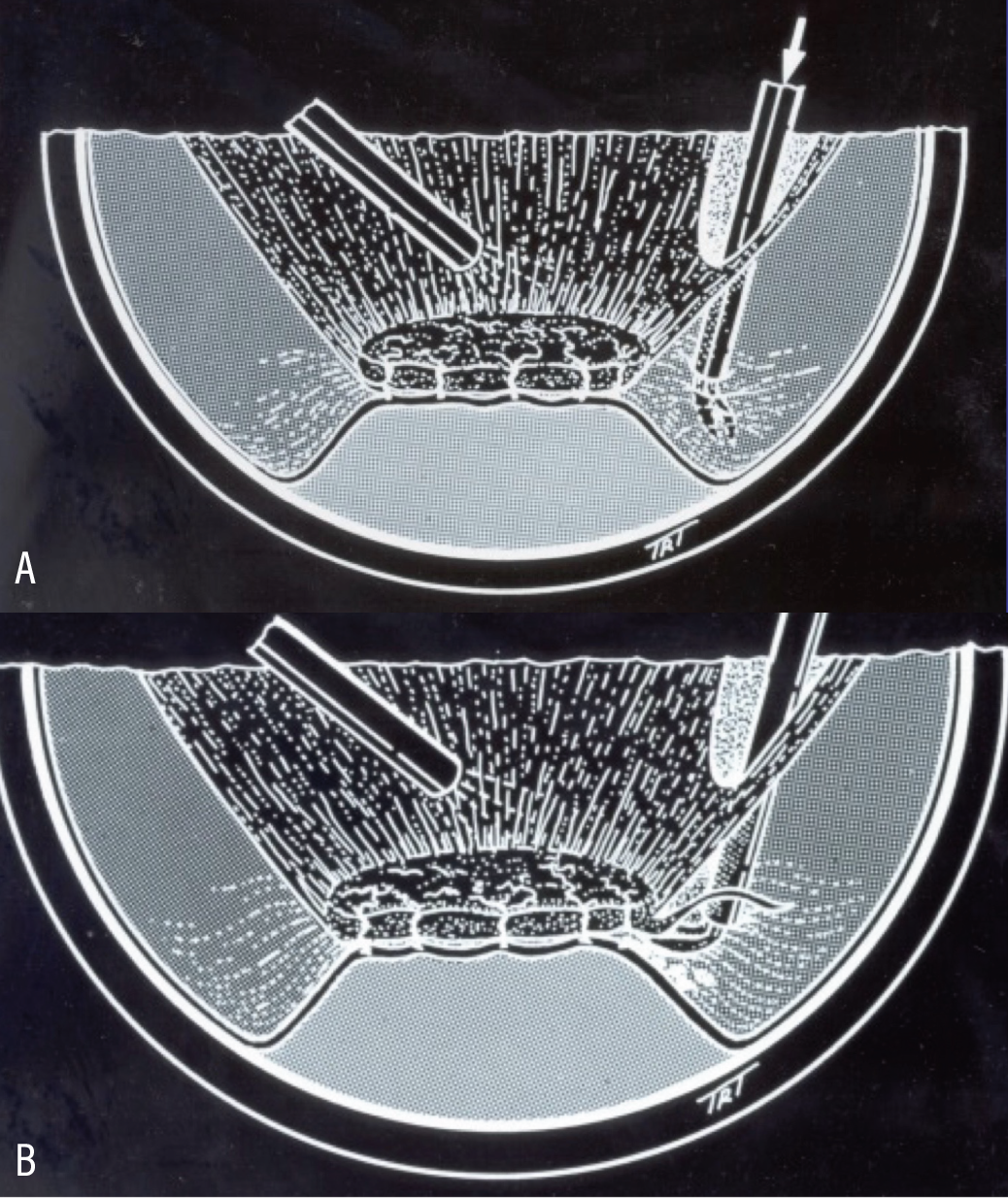 |
| Figure 1 . Instrumentation positioned between the posterior cortical vitreous and the retina via hyaloidotomy (A). Accurate dissection at the vitreoretinal interface helps to decrease the risk for iatrogenic retinal breaks (B). |
Funduscopic examination should be complete. Particular attention to vitreoretinal relationships, extent and location of fibrovascular proliferation, vitreoretinal traction, retinal breaks, areas of laser treatment and extent of retinal detachment is essential. Posterior vitreous detachment may or may not be present or complete; the PVD status will influence the complexity of the surgical dissection. Examination may be limited due to the presence of hemorrhage in various locations and will influence surgical planning and execution.
Cases that may not require surgery on initial evaluation should be followed carefully, particularly in the setting of recent anti-VEGF injections or panretinal photocoagulation. Progressive tractional changes may lead to vision-altering vitreous hemorrhage or macular detachment that may then warrant more expedited surgical intervention.
Imaging
Multimodal imaging is critical in the evaluation, surgical planning and postoperative surveillance of patients undergoing diabetic TRD repair. The importance of assessment, mapping, and documentation can’t be overstated. Preoperative widefield imaging, including fluorescein angiography should be performed to assess both central and peripheral perfusion status and location of fibrovascular proliferation. OCT assessment of the vitreoretinal interface including the presence, absence or location of PVD, TRD, macular detachment and/or macular edema will impact surgical success. OCT biomarkers for macular ischemia including disorganization of retinal inner layers (DRIL) can augment decision making and aid in patient discussions regarding visual prognosis.3 OCT-angiography may also be a useful adjunct in the evaluation of macular ischemia and can provide more nuanced data on deep capillary pathophysiology.4 When vitreous hemorrhage or other media opacities obscure direct funduscopic evaluation, B-scan ultrasonography must be performed to understand the anatomy prior to surgical intervention.5
• Don’t forget the fellow eye. Fellow eye status is fundamental to surgical decision making. Patients with diabetic TRD often have vision-threatening disease in the fellow eye. Every effort should be undertaken to stabilize disease in the fellow eye, typically with the use of anti-VEGF injections and PRP. For patients who are photophobic or otherwise intolerant of PRP in the clinic, consider PRP with an indirect laser in the operating room while the patient’s anxiety, cooperation and pain can be controlled. While disease severity between the two eyes may be asymmetric, if there’s significant asymmetry of disease between the two eyes, other factors such as the presence of retinal vein occlusion, other retinal vascular occlusive disease or carotid occlusive disease should be assessed. In some instances, treating the better eye first may be indicated.6
Surgical technique
Here are several pearls for operating on these cases:
• Pars plana vitrectomy: A step-wise approach. When performing pars plana vitrectomy for TRD repair, the surgeon must first decide which gauge instrumentation to use. Larger gauge instrumentation (23 ga) is more efficient, especially for the core vitrectomy. Smaller gauge vitrectomy (27 ga) allows for more precise segmentation and delamination, allowing the surgeon to fit the cutter into tighter spaces and potentially decreasing the risk of iatrogenic retinal breaks.7 Our approach is to use 25-ga instrumentation and a hybrid approach with a 27-ga cutter as needed for tighter dissections.
Every TRD case has unique features. However, having a general framework for TRD repair can be useful. Our intraoperative approach typically involves media clearance, including core vitrectomy, confirmation of PVD location if present, hyaloidotomy, 360-degree segmentation of the anterior and posterior cortical vitreous and relief of vitreoretinal interface pathology whether via segmentation and/or delamination techniques. Particular attention to dissect within the correct surgical plane helps prevent iatrogenic hemorrhage and retinal breaks (See Figure 1). PVD induction, if necessary, should be managed with extreme care, avoiding undue traction on fibroproliferative pathology.8
• Intraoperative visualization for ideal dissection. Fine dissection of fibrovascular membranes requires a clear view, and the surgeon should make every effort to optimize visualization. This includes the use of pupil expanders and removal of a visually significant cataract when necessary. Visualization of the posterior cortical vitreous is critical for successful TRD repair.
Diabetic vitreous is often schitic, and intraoperative identification of this nuanced finding can simplify and dramatically improve surgical success.9 Dissection of the thin layer of vitreous attached to the retina posterior to the apex of the TRD facilitates access to the correct surgical plane. Triamcinolone can be a useful adjuvant. Our approach is to dilute triamcinolone 1:4 with balanced salt solution such that a slow, low-turbulence injection can reliably achieve a light dusting of the residual vitreous without obscuring the view with excess drug. Other adjuncts for chromovitrectomy include staining with trypan blue or Brilliant Blue G.
• Hemostasis. Throughout TRD surgery, and particularly during fibrovascular membrane dissection, bleeding may occur. In general, all intraoperative bleeding should be promptly addressed with endodiathermy or laser. If tamponade with elevated IOP is necessary, we recommend observing the lesion with the diathermy probe near the lesion while slowly lowering the IOP. If the lesion remains hemostatic, the dissection can recommence. If hemorrhage recurs, it can be immediately controlled with additional treatment. Diabetic blood is rich in fibrin and extensive hemorrhage can be highly adherent to the retina requiring additional work to dissect the hemorrhage off the retinal surface. In general, avoid prolonged elevation of intraoperative pressure as this may further compromise capillary perfusion and exacerbate corneal edema, hampering visualization.10
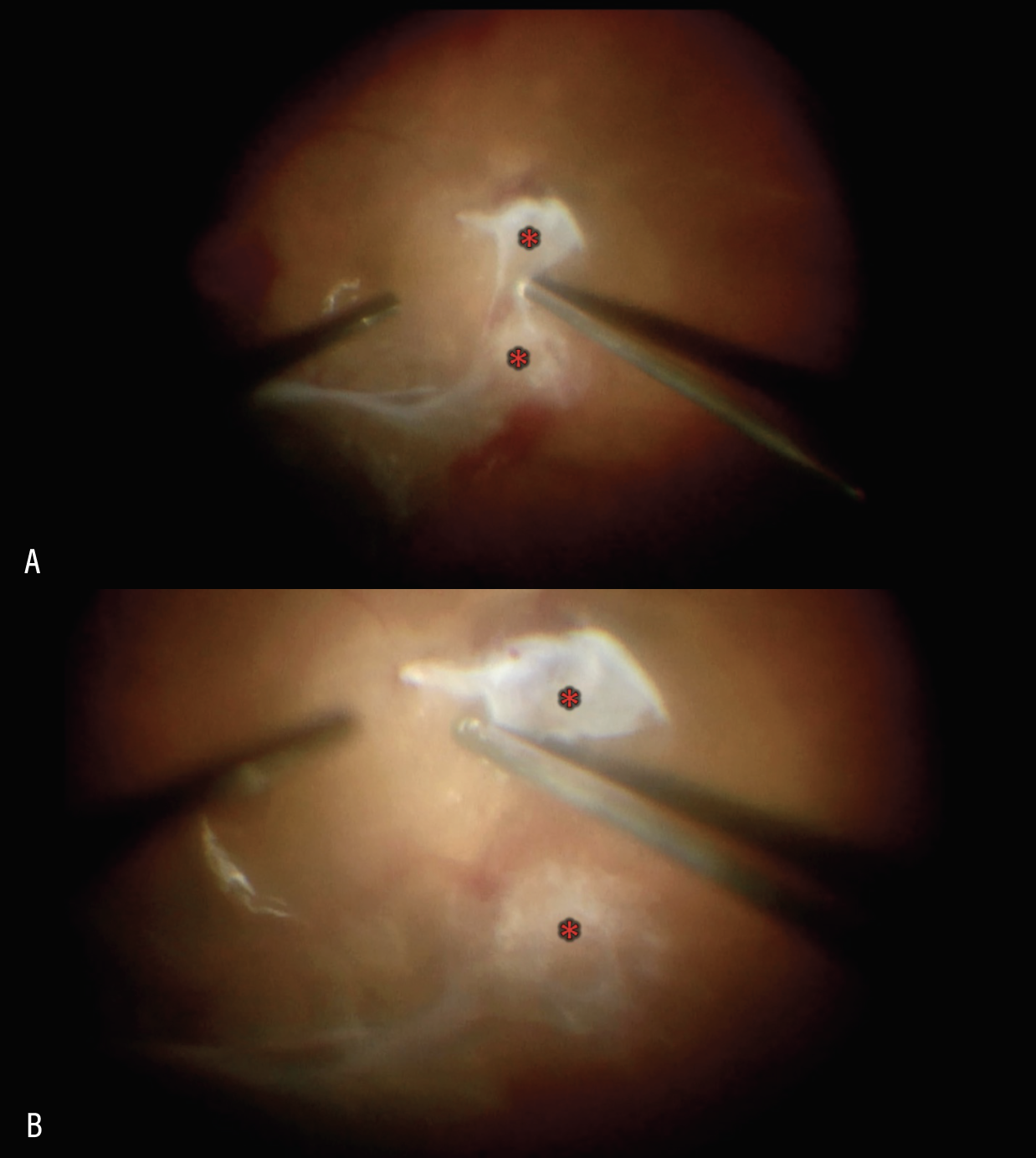 |
| Figure 2. Segmentation of two adjacent areas of proliferation (red asterisk) using the vitreous cutter positioned in the vitreoretinal interface (A). Two islands of fibrovascular proliferation (red asterisk) following segmentation (B). |
Following complete dissection, we like to lower the IOP to approximately 10 mmHg and observe the retinal surface to identify and address any small oozing vessels that may result in postoperative vitreous hemorrhage. Furthermore, a complete air-fluid exchange at the end of surgery may encourage coagulation of blood at persistent oozing sites, minimizing the extent of postoperative hemorrhage and hastening visual recovery. Pay close attention to sclerotomy wound integrity to avoid transient hypotony in the postoperative period.
• Segmentation and delamination: How much is enough? One main goal of TRD repair is to safely relieve all vitreoretinal traction, especially traction affecting the macula. While it’s typically recommended to attempt complete removal of all fibrovascular proliferation, this must be weighed against the risk of creating iatrogenic retinal breaks that can compromise surgical success. Two techniques are generally employed for management of traction. One is segmentation, whereby fibrovascular proliferative tissue is segmented or isolated as islands of tissue from adjacent areas of proliferation. These tissue remnants are surgically separated from adjacent proliferative tissue without accessing and dissecting them from the retinal surface (See Figure 2). This maneuver is considered to carry less risk of creating retinal breaks but more risk of late hemorrhage or retinal folds from persistent traction.11
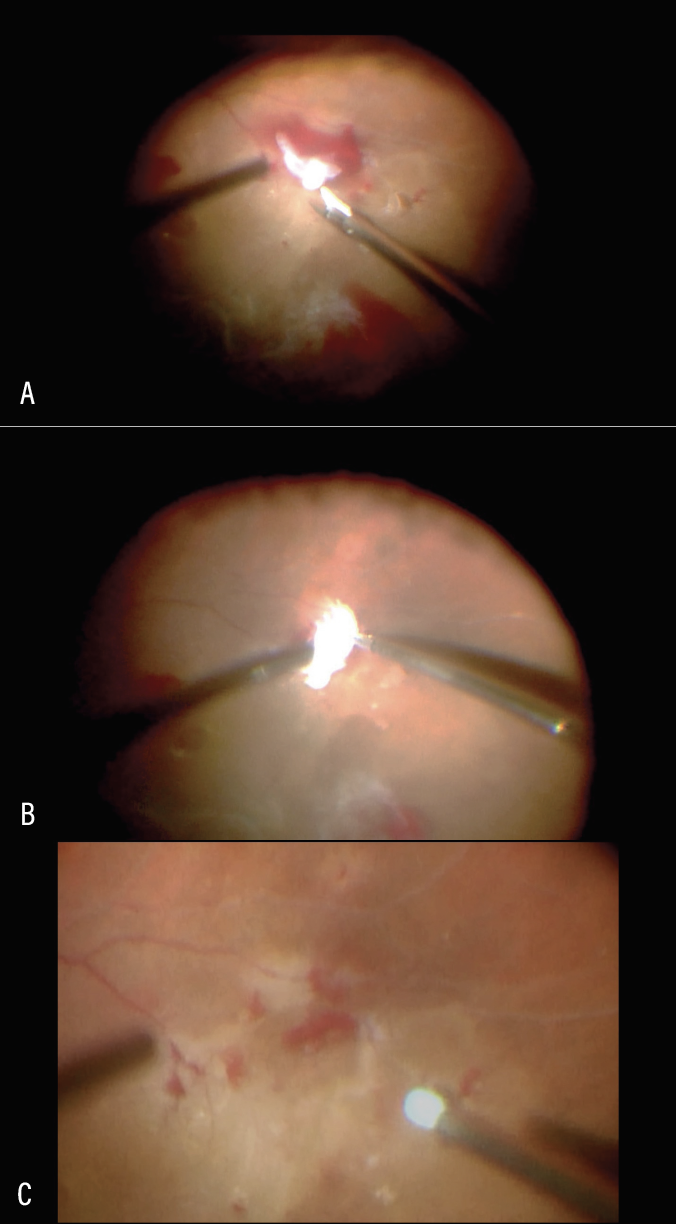 |
| Figure 3. Delamination of fibrovascular membrane using horizontal scissors (A). Sharp dissection under fibrovascular proliferation to cut the vascular peg (B). Delamination complete. Residual hemorrhage removed with soft tip cannula (C). |
Delamination typically involves complete removal of fibrovascular membranes by dissecting in the vitreoretinal interface and sharply transecting the fibrovascular stalks or pegs emanating from the retina into the posterior cortical gel (Figure 3). Delamination can be performed with the vitreous cutter, horizontal or vertical scissors, or other instruments.
Bimanual surgery using hands-free chandelier illumination allows the surgeon’s second hand to elevate the posterior cortical gel or fibrovascular membranes with forceps, facilitating sharp dissection of vascular pegs within the preretinal space. Delamination requires access to the correct surgical plane; instrumentation should be positioned between the posterior hyaloid and the retina to avoid iatrogenic retinal breaks.
The configuration of diabetic TRD can range from a “tabletop” detachment where the entire area of detachment is elevated evenly like a table with the legs representing the fibrovascular pegs to a very uneven “mountain range” configuration where detached retina is unevenly elevated above both discrete and broad areas of vitreoretinal adhesion. Preoperative evaluation and mapping with examination and imaging are critical when planning for this type of approach. Traditionally, the delamination technique is thought to create a more complete relief of traction but carries with it a higher risk of retinal breaks. Once a break is encountered, all associated traction must be relieved. Residual traction, even with a break that has been lasered, typically yields recurrent RD. Residual subretinal fluid in the absence of retinal breaks doesn’t need to be drained.12
• Panretinal photocoagulation. Preoperative PRP should be considered if active proliferation is present.13 This indication is magnified when no PVD is detectable. Repairing a diabetic TRD when the posterior cortical vitreous is completely attached can be particularly difficult and dangerous. PRP can promote a slowly progressive PVD and optimize surgical execution.
Intraoperatively PRP is fundamental to successful outcomes. The best time to perform PRP is when visualization and access to the peripheral retina are optimized. We typically recommend PRP placement following complete vitrectomy and membrane dissection. Preoperative angiography or even intraoperative angiography can be used to guide the extent of PRP, but generally we recommend PRP extending anteriorly to the ora serrata with scleral depression or laser under air if needed (Figure 4). Some surgeons prefer to extend PRP to a posterior margin within a disc diameter or two from the nerve and vascular arcades. Others recommend a more equatorial posterior margin to both preserve visual field and avoid treating areas of retina that may still be detached. Posterior fill-in PRP is far simpler than anterior fill-in and can be achieved in the office without much difficulty for patient or surgeon.
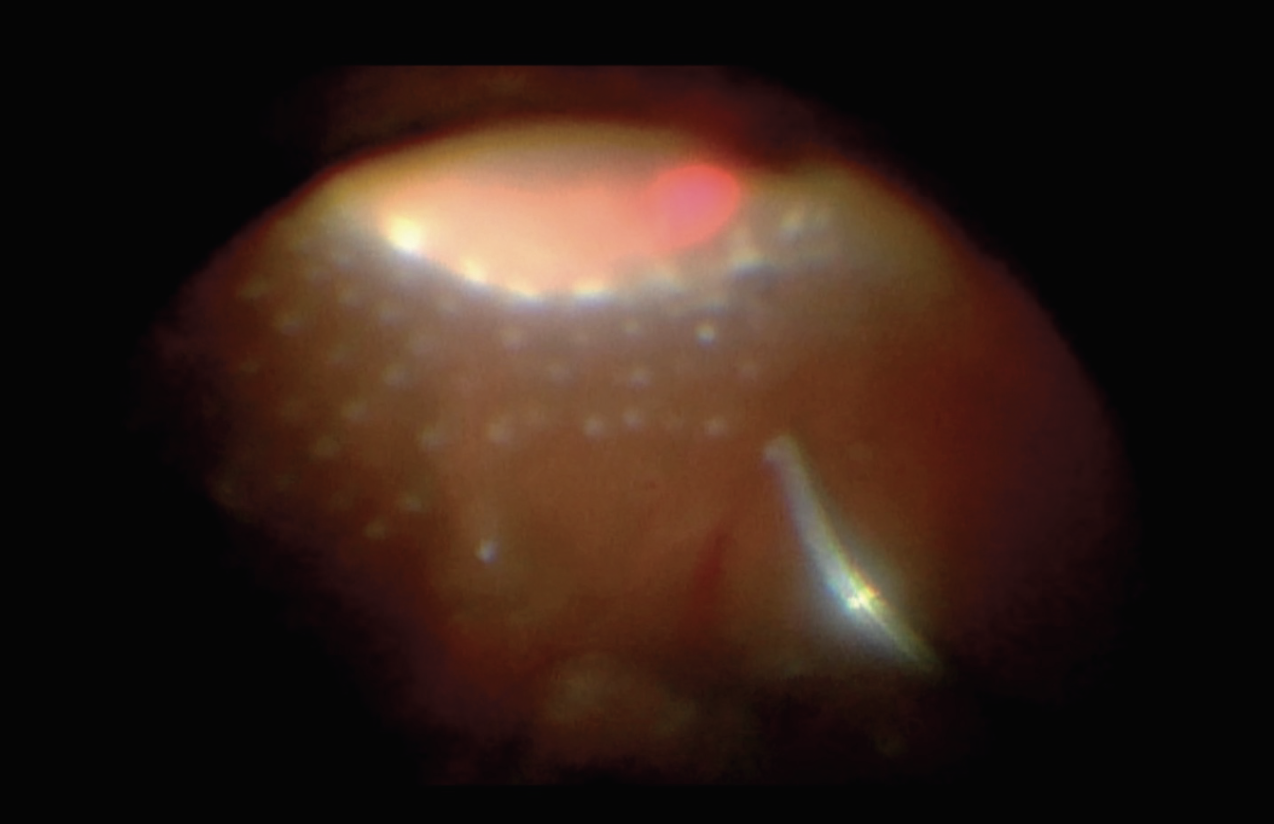 |
| Figure 4. Anterior panretinal photocoagulation applied with the assistance of scleral depression. |
• What about anti-VEGF? Preoperative anti-VEGF injections can be helpful to minimize intraoperative and postoperative hemorrhage. Timing and dose can be important considerations depending on the severity of the TRD. Continuous exposure to preoperative injections raises the risk of fibrovascular membrane contraction (the so-called “crunch” phenomenon) that can exacerbate tractional retinal detachment or even convert TRD to combined rhegmatogenous-TRD.14 Delays in surgery may exacerbate the underlying pathology and compromise surgical outcomes. Therefore, when performing preoperative anti-VEGF injections, it’s important that the patient has been medically cleared for surgery. Some surgeons also prefer intravitreal injection of anti-VEGF medications at the end of surgery to decrease the risk of postoperative hemorrhage in theory.
Postoperative care
Postoperative complications following TRD repair include “shake-out” or postoperative vitreous hemorrhage, ocular hypertension and new or recurrent retinal detachment. Most cases of postoperative hemorrhage can be observed, although severe hemorrhage may require repeat surgery or in-office air-fluid exchange. Ocular hypertension should be managed aggressively with IOP-lowering medications and co-management with a glaucoma specialist is often recommended.15
Note that persistent subretinal fluid following TRD repair is common and may require months to resolve. OCT imaging can help to objectively quantify the amount of subretinal fluid and serial imaging should demonstrate resolution of fluid over time. Worsening fluid raises the concern for an occult retinal break that may require further surgical intervention.
In conclusion, repairing diabetic TRDs is a complex endeavor that requires a multifaceted approach. By understanding the pathophysiology, employing meticulous surgical techniques, and ensuring comprehensive pre- and postoperative care, surgeons can optimize outcomes for patients suffering from this potentially blinding condition. Each case is unique, and thoughtful consideration of individual patient factors will lead to the best possible outcomes. Ultimately, the goal is not only to repair the detachment but to preserve and restore vision, allowing patients to maintain their independence and quality of life. RS
REFERENCES
1. Berrocal MH, Acaba LA, Acaba A. Surgery for diabetic eye complications. Curr Diab Rep. 2016;16:10:99.
2. Au A, Bajar BT, Wong BM, Daskivich LP, Hosseini H, Prasad PS. Systemic and ocular outcomes in patients with young-onset type 2 diabetes. J Diabetes Complications. 2024;38:2:108670.
3. Liao A, Fortes B, Au A, Hou K. Management of diabetic tractional retinal detachments: Surgical experiences from a large tertiary academic institution. Curr Opin Ophthalmol. 2025;36:3:177-181.
4. Russell JF, Scott NL, Townsend JH, Shi Y, Gregori G, Crane AM, Flynn HW Jr, Sridhar J, Rosenfeld PJ. Wide-field swept-source optical coherence tomography angiography of diabetic tractional retinal detachments before and after surgical repair. Retina. 2021;41:8:1587-1596.
5. Demir G, Arici M, Alkin Z. Preoperative evaluation of tractional retinal detachment with B-mode ultrasonography in diabetic vitreous hemorrhage. Beyoglu Eye J. 2021;6:1:49-53.
6. Hwang JC, Sharma AG, Eliott D. Fellow eye vitrectomy for proliferative diabetic retinopathy in an inner city population. Br J Ophthalmol. 2013;97:3:297-301.
7. Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, Tano Y. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009.
8. Stewart MW, Browning DJ, Landers MB. Current management of diabetic tractional retinal detachments. Indian J Ophthalmol. 2018;66:12:1751-1762.
9. Schwatz SD, Alexander R, Hiscott P, Gregor ZJ. Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology. 1996;103:2:323-8.
10. Varshney T, Babu KN, Elamurugan V, Krishnan M. Haemostasis during diabetic vitrectomy. Indian J Ophthalmol. 2023;71:10:3416-3417.
11. Cruz-Iñigo YJ, Acabá LA, Berrocal MH. Surgical management of retinal diseases: Proliferative diabetic retinopathy and traction retinal detachment. Dev Ophthalmol. 2014;54:196-203.
12. Iyer SSR, Regan KA, Burnham JM, Chen CJ. Surgical management of diabetic tractional retinal detachments. Surv Ophthalmol. 2019;64:6:780-809.
13. Patel V, Rohowetz LJ, Pakravan P, Kalavar M, Yannuzzi NA, Sridhar J. Outcomes of pars plana vitrectomy with panretinal photocoagulation for treatment of proliferative diabetic retinopathy without retinal detachment: A seven-year retrospective study. Clin Ophthalmol. 2023;17:471-478.
14. Tan Y, Fukutomi A, Sun MT, Durkin S, Gilhotra J, Chan WO. Anti-VEGF crunch syndrome in proliferative diabetic retinopathy: A review. Surv Ophthalmol. 2021;66:6:926-932.
15. Yau GL, Silva PS, Arrigg PG, Sun JK. Postoperative complications of pars plana vitrectomy for diabetic retinal disease. Semin Ophthalmol. 2018;33:1:126-133.



