Take-home points
|
 |
Bios Dr. Rohowetz is a vitreoretinal surgery fellow at Bascom Palmer Eye Institute, University of Miami Miller School of Medicine. Dr. Flynn is the J. Donald M. Gass Distinguished Chair in Ophthalmology at the University of Miami’s Miller School of Medicine, and a professor of ophthalmology at Bascom Palmer. Disclosures: None |
Diabetic retinopathy remains one of the leading causes of vision impairment and blindness in working-age adults worldwide.1 As the global burden of diabetes increases, retina specialists are tasked with not only treating the sight-threatening complications of DR but also detecting and monitoring the disease earlier and more effectively. Optical coherence tomography, with its ability to provide high-resolution cross-sectional images of the retina, has become a cornerstone in the evaluation and management of DR. From identifying subclinical changes to guiding individualized treatment regimens, OCT plays a pivotal role in improving patient outcomes and enhancing clinical decision-making. Here, we’ll review OCT’s capabilities in this area, as well as take a look at what benefits newer technologies might bring.
Early Detection and Risk Stratification
Traditional staging systems for diabetic retinopathy, such as the Early Treatment Diabetic Retinopathy Study classification, rely heavily on fundus photography and clinical examination. While these tools remain fundamental, they often miss early structural changes, particularly those affecting the macula. Optical coherence tomography enables the detection of subtle alterations such as thickening of the retina, early intraretinal cysts or signs of neuroretinal dysfunction before they’re visible clinically. Indeed, OCT has been an important tool used in the Diabetic Retinopathy Clinical Research Network trials.2,3
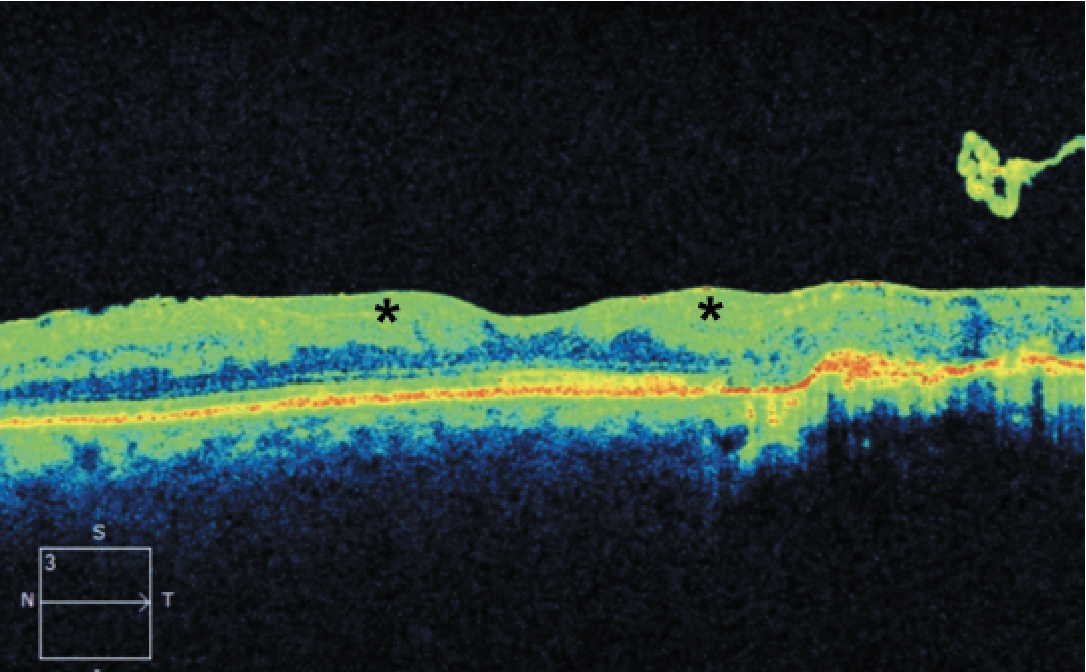 |
| Figure 1. Optical coherence tomography of a 41-year-old male with a history of proliferative diabetic retinopathy demonstrating disorganization of the retinal inner layers (asterisks). |
More specific pathologic changes such as disorganization of the retinal inner layers (DRIL; Figure 1) can be appreciated only through OCT and have been identified as markers of visual potential and predictors of DR progression.4 Similarly, outer retinal disruption (Figure 2) and early photoreceptor damage may indicate more advanced disease with poor visual prognosis.5
By incorporating OCT findings and these evolving technologies into the clinical workflow, retina specialists can identify patients at higher risk of progression, allowing for earlier intervention and more tailored follow-up intervals. This is particularly useful in patients with diabetes who do not yet meet criteria for treatment but warrant closer surveillance.
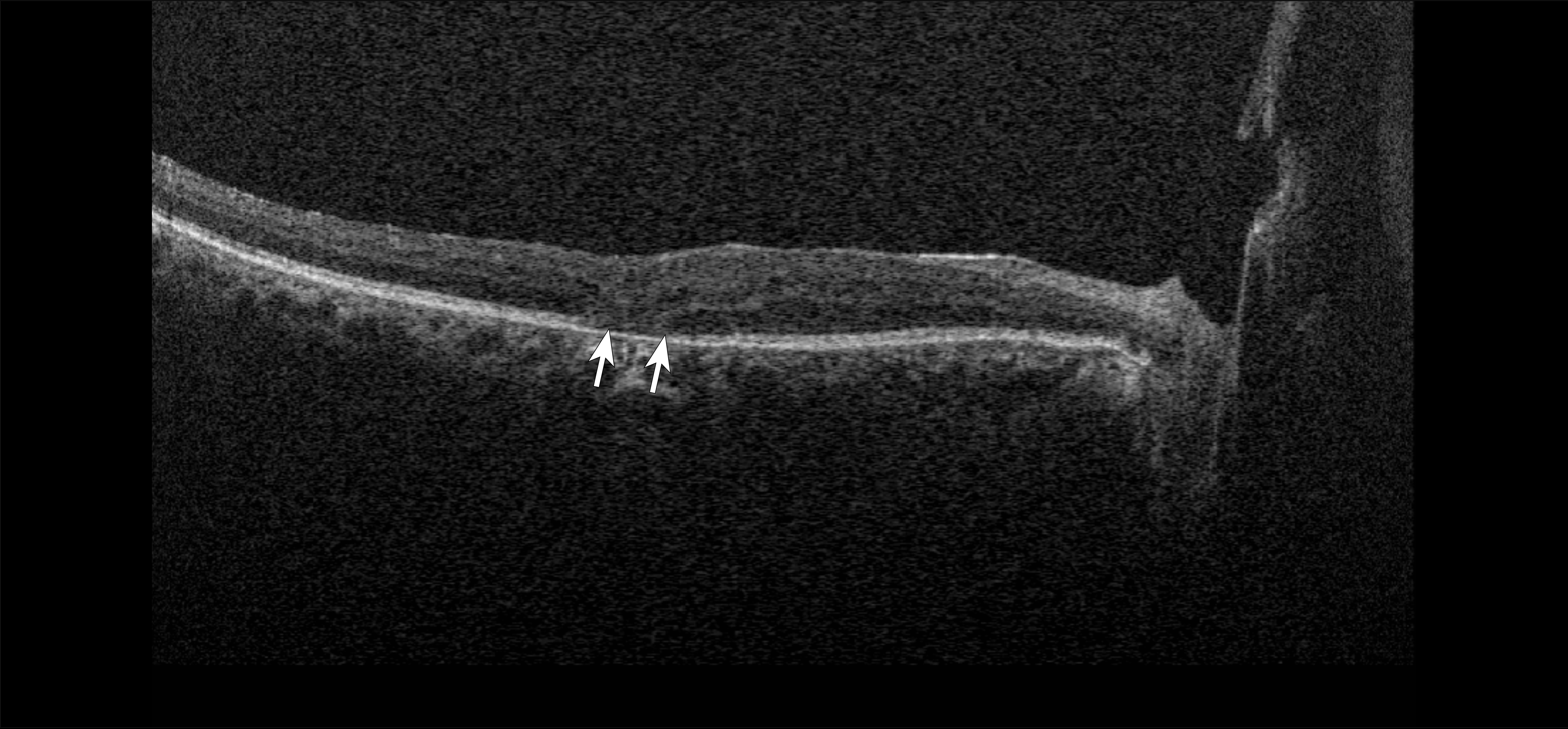 |
| Figure 2. Optical coherence tomography of a 59-year-old with a history of proliferative diabetic retinopathy demonstrating outer retinal disruption (arrows). |
Monitoring diabetic macular edema
One of the most impactful contributions of OCT to diabetic eye care has been in the management of diabetic macular edema, the leading cause of vision loss in DR.6 While ophthalmoscopy can detect clinically significant macular edema, OCT provides a far more sensitive and quantitative assessment of retinal thickening and the presence of intraretinal or subretinal fluid.7
Optical coherence tomography enables precise measurement of central subfield thickness, which is often used as a surrogate endpoint in clinical trials and treatment protocols. It also allows for the characterization of fluid patterns and identification of structural features such as hyperreflective foci or hard exudates.
These features not only guide initial treatment decisions but also help retina specialists assess disease activity over time. For instance, persistent intraretinal fluid despite multiple anti-VEGF injections may prompt a switch to an alternative agent while resolution of fluid may justify extension of treatment intervals.8
Artificial intelligence–assisted OCT interpretation is also emerging as a tool for earlier and more standardized detection of DME. AI algorithms trained on large imaging datasets can identify patterns of disease activity, flag subtle anatomic changes, and potentially support more consistent and proactive management.9 In parallel, home-based OCT platforms are being developed to enable remote monitoring of patients with macular disease such as DME. These devices may allow for more frequent assessments, earlier detection of fluid recurrence and timely treatment adjustments, particularly beneficial for patients who face challenges with frequent clinic visits.10
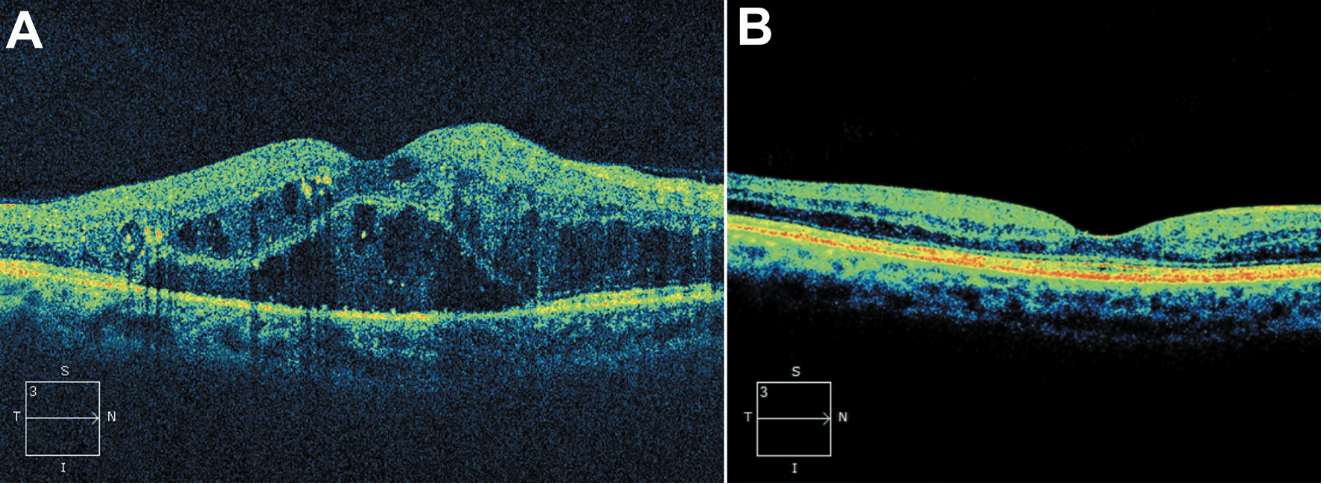 |
| Figure 3. (A) Optical coherence tomography of a 47-year-old male with a history of nonproliferative diabetic retinopathy demonstrating the presence of subretinal fluid, intraretinal fluid and intraretinal deposits. (B) Optical coherence tomography six years after initiation of regular intravitreal anti-VEGF therapy demonstrating resolution of intraretinal and subretinal fluid. |
Furthermore, OCT imaging facilitates shared decision-making with patients, who can visualize the impact of their therapy and understand the importance of treatment adherence. The ability to track objective improvements in retinal architecture can enhance patient engagement and satisfaction (Figure 3).
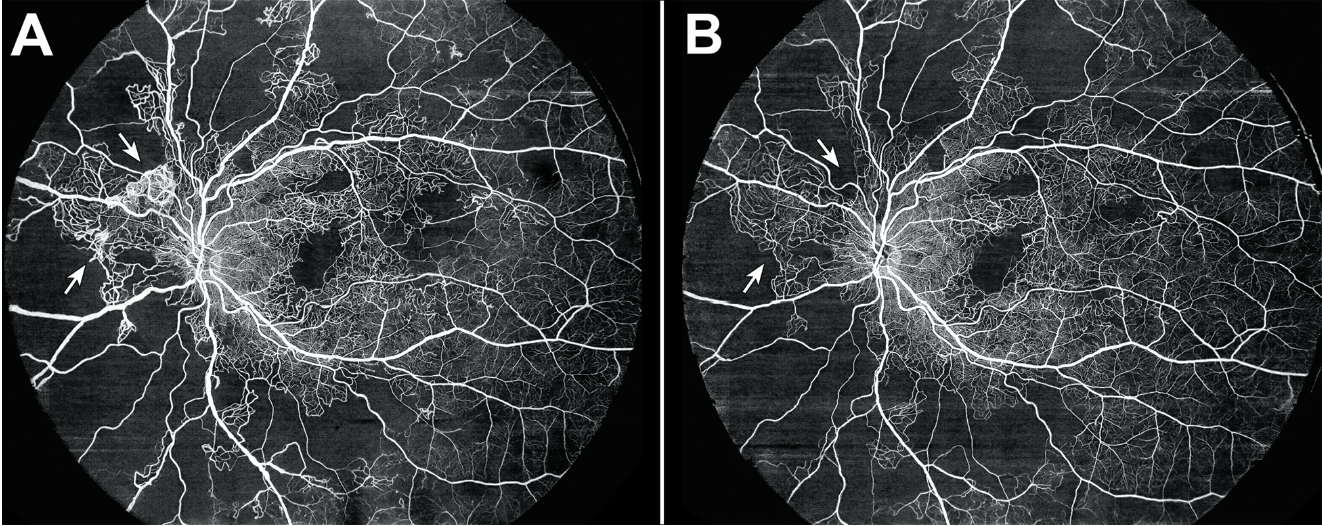 |
|
Figure 4. (A) Optical coherence tomography angiography of a 40-year-old female with a history of proliferative diabetic retinopathy demonstrating capillary nonperfusion and peripapillary neovascularization elsewhere (arrows). (B) Optical coherence tomography angiography one year after treatment with intravitreal bevacizumab demonstrating regression of the neovascularization (arrows). |
Assessing treatment response and guiding individualized therapy
The era of one-size-fits-all therapy in diabetic retinopathy is evolving toward a more personalized approach, and OCT is at the heart of this shift. Response to intravitreal therapy is highly variable, and structural outcomes on OCT often correlate imperfectly with changes in visual acuity. However, certain OCT biomarkers, such as the presence of DRIL, ellipsoid zone disruption or persistent cystoid spaces, can offer prognostic insights that help guide long-term management.4,11 For example, in patients undergoing anti-VEGF therapy, OCT is invaluable for determining retreatment intervals under treat-and-extend or PRN regimens. Evidence suggests that patients with persistent or recalcitrant fluid respond better to shorter intervals, whereas stable patients can often tolerate extension.12 Corticosteroid agents including dexamethasone implants, fluocinolone implants and triamcinolone acetonide offer alternative strategies for chronic DME, and OCT monitoring can help determine treatment response and optimal intervals.13
In surgical contexts, OCT can also aid in planning by identifying tractional elements or vitreomacular adhesion that may necessitate vitrectomy. Postoperatively, serial OCT scans can document resolution of edema and retinal reattachment, allowing for a more objective assessment of surgical outcomes.
OCT angiography: A new frontier in DR evaluation
More recently, OCT angiography has emerged as a powerful adjunct in the evaluation of diabetic retinopathy. This noninvasive imaging modality captures motion contrast from blood flow, providing a detailed view of retinal and choroidal microvasculature without the need for fluorescein dye.14
Optical coherence tomography angiography allows clinicians to identify areas of capillary nonperfusion, microaneurysms and enlargement of the foveal avascular zone—hallmarks of ischemia that are closely linked to disease severity.15 Unlike fluorescein angiography, OCTA can be repeated frequently and noninvasively, making it a valuable tool for longitudinal monitoring.
In eyes with nonproliferative DR, OCTA can detect progressive microvascular changes even in the absence of overt clinical signs, potentially identifying patients who may benefit from closer observation or early intervention.16 In proliferative disease, OCTA can complement ophthalmoscopy and widefield imaging by delineating neovascular networks and their response to panretinal photocoagulation or anti-VEGF therapy (Figure 4).17
While traditional OCTA does have limitations—such as a smaller field of view compared to ultra-widefield FA and vulnerability to motion artifacts—it represents a promising step toward more comprehensive, noninvasive retinal vascular imaging.
Surgical planning and postoperative evaluation
Though many patients with diabetic retinopathy are managed medically, surgical intervention remains an option for complications such as non-clearing vitreous hemorrhage, tractional retinal detachment and dense epiretinal membranes. Optical coherence tomography provides essential preoperative information in these cases, particularly in identifying signs of progression or foveal involvement.18
Postoperatively, OCT can be used to monitor retinal architecture, detect recurrent or residual edema, and assess the integrity of the ellipsoid zone and other photoreceptor layers. This information can be critical in counseling patients regarding visual prognosis and planning adjunctive treatments if needed.
Limitations and considerations
Despite its many advantages, OCT isn’t without limitations. Image artifacts can result from media opacities, patient movement or segmentation errors, which may lead to misinterpretation. Moreover, traditional OCT generally provides only a limited view of the peripheral retina, where proliferative changes and ischemia may occur outside the macula.19 However, new widefield technologies are being developed to improve image quality and expand the field of view.
It’s also important to remember that OCT is an adjunct—not a replacement—for clinical examination and functional testing. In some cases, visual acuity changes may precede structural changes on OCT, underscoring the need for comprehensive assessment.
Cost, access and reimbursement also play a role, particularly in practices that serve underserved populations or operate in resource-limited settings. As OCT and OCTA are bundled with some evaluation codes or imaging services, it’s not always separately reimbursed, and this may influence usage patterns in clinical practice.
Bottom line
Optical coherence tomography has transformed the way retina specialists diagnose, monitor and treat diabetic retinopathy. Its ability to detect subtle structural changes, quantify disease activity, and guide individualized therapy has elevated the standard of care and improved outcomes for millions of patients. As OCT technology continues to evolve—with advances such as swept-source OCT, AI-assisted interpretation and home-based monitoring devices—the future of diabetic eye care will be even more precise, personalized and proactive. By integrating OCT into a comprehensive management strategy, retina specialists can stay one step ahead of diabetic retinopathy and preserve vision in a growing population at risk. RS
REFERENCES
1. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:3:556-564
2. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:6:1351-1359
3. Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: A randomized clinical trial. JAMA. 2019;321:19:1880-1894
4. Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:11:1309-1316
5. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:6:688-693
6. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:13:1193-1203
7. Browning DJ, McOwen MD, Bowen RM Jr, O’Marah TL. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology. 2004;111:4:712-715
8. Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: Two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:9:1533-1538.
9. Li L, Zhang W, Tu X, et al. Application of artificial intelligence in precision medicine for diabetic macular edema. Asia Pac J Ophthalmol (Phila). 2023;12:5:486-494.
10. Blinder KJ, Calhoun C, Maguire MG, et al. Home OCT imaging for newly diagnosed neovascular age-related macular degeneration: A feasibility study. Ophthalmol Retina. 2024;8:4:376-387.
11. Borrelli E, Grosso D, Barresi C, et al. Long-term visual outcomes and morphologic biomarkers of vision loss in eyes with diabetic macular edema treated with anti-VEGF therapy. Am J Ophthalmol. 2022;235:80-89
12. Lim SY, Wong WM, Seah I, et al. Treat and extend regimen for diabetic macular oedema—A systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2023;261:2:303-315
13. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:10:1904-1914
14. Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:1:45-50
15. Waheed NK, Rosen RB, Jia Y, et al. Optical coherence tomography angiography in diabetic retinopathy. Prog Retin Eye Res. 2023;97:101206
16. Durbin MK, An L, Shemonski ND, et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA. Ophthalmol 2017;135:4:370-376
17. Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160:1:35-44 e31
18. Oellers P, Mahmoud TH. Surgery for proliferative diabetic retinopathy: new tips and tricks. J Ophthalmic Vis Res. 2016;11:1:93-99
19. Blodi BA, Domalpally A, Tjaden AH, et al. Comparison of ETDRS 7-field to 4-widefield digital imaging in the evaluation of diabetic retinopathy severity. Transl Vis Sci Technol. 2022;11:1:13



