Take-home points
|
 |
Bios Dr. Carrillo is a medical scribe at the Palo Alto Medical Foundation. Dr. Alsoudi is an ophthalmology resident focusing on surgical retina and ocular oncology at Baylor College of Medicine, Houston. Dr. Rahimy is a vitreoretinal specialist at Palo Alto Medical Foundation as well as an adjunct clinical assistant professor of ophthalmology at Byers Eye Institute at Stanford University, both in Palo Alto, California. Disclosures for Dr. Rahimy: Consultant: AbbVie/Allergan, Alcon, Apellis, Genentech, Harrow, Regeneron, Zeiss; Speaker: Abbvie/Allergan, Apellis, Genentech, Regeneron |
Established risk factors for the development and progression of DR, a leading cause of vision loss, 1 include poor glycemic control, hypertension, hyperlipidemia and tobacco use/nicotine dependence.2,3 In recent years, however, obstructive sleep apnea has emerged as a potentiating risk factor for the progression of DR to more advanced stages, resulting in greater visual impairment and an increased treatment burden.2 Recognition of OSA as an underlying risk factor presents an opportunity for physicians to improve ocular as well as systemic outcomes for patients with DR by properly screening for potential OSA and ensuring timely referral and proper management of this condition. Here, we review existing evidence on DR progression rates in patients with and without OSA, highlighting a recent aggregate, electronic health record (EHR)-based study on the progression of non-proliferative DR over a five-year period.
Current understanding of OSA and DR
Obstructive sleep apnea is a chronic breathing disorder that causes cessation of airflow due to an obstruction of the upper airway during sleep.4 There’s an established association between diabetes and OSA, with OSA affecting 58 to 86 percent of individuals with diabetes.2,5 OSA is also associated with other ophthalmic conditions, specifically in the retina, including retinal vein occlusion and central serous chorioretinopathy.6,7,8
More recently, emerging evidence has supported a positive association between OSA and DR. In one study, researchers evaluated diagnoses in 317 patients and found that severe OSA is associated with both prevalence and severity of DR.9 Patients with severe OSA were found to be at greater risk of developing DR, PDR and DME compared to patients with mild-to-moderate OSA. Another investigation evaluated 170 patients with type 2 diabetes and concurrent OSA of varying severity.10 Findings from this group included a higher prevalence of DR in patients with severe OSA than in patients with mild-to-moderate OSA. However, a separate study with a larger cohort of 556 patients didn’t find a significant difference in DR prevalence when comparing patients with mild OSA and moderate-severe OSA.11
The overlapping relationship between OSA and DR may be potentiated by the shared risk factors, associated comorbidities and underlying pathophysiological commonalities exerted within the retinal microvasculature. Comorbidities of OSA—hypertension, hyperlipidemia, CAD, obesity and hypothyroidism—are also potential risk factors for DR progression and vice versa, creating a complex, interwoven relationship between the two conditions.2 How studies choose to mitigate and interpret the confounding impact of this overlap may result in different observed effects of OSA on DR. This presents a challenge in establishing a direct causal relationship between OSA and DR.
Within the retina, OSA may adversely impact tissue structural integrity, microcirculation, resultant tissue ischemia and release of pro-angiogenic factors, all of which are associated with onset of PDR and macular edema,12 including retinal manifestations. Literature highlighted the clear association between OSA and numerous ocular conditions including glaucoma and papilledema. This comprehensive and narrative review aims to summarize up-to-date clinical research concerning the association of OSA and vascular conditions that affect the retina. OSA is associated with central serous chorioretinopathy. It's also been recognized as an independent contributing factor to the development of insulin resistance, subsequently leading to poor glucose regulation.13 It’s therefore hypothesized that OSA may have a greater impact on the progression of DR to more advanced forms compared to the prevalence of DR in diabetic patients without concurrent OSA.
 |
OSA as a risk factor for DR progression
A recent large-scale retrospective cohort study investigated the progression of DR over a five-year period in patients with NPDR divided into two study groups: 11,931 patients with OSA and 11,931 matched individuals without OSA.2 The study used a multicenter aggregate EHR research network, TriNetX, which encompasses more than 300 million patient lives worldwide, including data from more than 64 U.S. institutions. Control group participants were selected for by propensity score matching to balance for various baseline characteristics, systemic comorbidities (i.e., hypertension, cardiovascular disease, renal disease), nicotine use/dependence, medication use (i.e., glucagon-like peptide-1 (GLP1-) agonists) and laboratory values (i.e. hemoglobin A1c (HbA1c), body mass index).
Long-term outcomes of NPDR patients in each group were compared during the course of the study period and measured at one, three and five years. Results of the study revealed that patients with OSA demonstrated significantly faster progression to PDR and DME from baseline NPDR. Progression to PDR was significantly higher in the cohort with concurrent OSA at all three time points (one-year RR: 1.34, p<0.001; three-year RR: 1.31, p<0.001; five-year RR: 1.28, p<0.001) as well as DME (one-year RR: 1.31, p<0.001; three-year RR: 1.19, p<0.001); five-year RR: 1.18, p<0.001). This translated to a higher need for treatment with intravitreal injections of anti-VEGFs (one-year RR: 1.59, p<0.001; three-year RR: 1.58, p<0.001; five-year RR: 1.54, p<0.001) and laser photocoagulation in this study group compared to patients without OSA. Furthermore, there was a significant difference observed in the baseline HbA1c levels, a known modifiable risk factor for DR, in patients with concurrent OSA and NPDR (7.96 ± 1.96 percent) versus those without OSA (8.07 ± 1.93 percent; p<0.001).
Patients with OSA were observed to have lower HbA1c levels at baseline but still experienced a higher rate of DR progression over five years. While the prevalence of diabetic tractional retinal detachments and resulting need for surgical intervention didn’t differ significantly between groups, authors attribute this to increased utilization of treatments that mitigate progression to end-stage DR.
Individuals with NPDR and OSA also experienced significantly higher rates of stroke (one-year RR: 1.80, p<0.001; three-year RR: 1.56, p<0.001; five-year RR: 1.49, p<0.001), myocardial infarction (one-year year RR: 1.51, p<0.001; three-year year RR: 1.46, p<0.001; five-year RR: 1.43, p<0.001), and mortality (one-year RR: 1.31, p<0.001; three-year RR: 1.19, p<0.001; five-year RR: 1.15, p<0.001) compared to those without OSA. These findings underscore the substantial impact of OSA on the course of DR and the health outcomes of patients.
Practical implications and current gaps in care
Increasing awareness of the relationship between OSA and DR may help address a current overreliance on traditional pharmacotherapy by emphasizing early co-management of OSA as a risk factor for DR progression. Typical treatment of OSA has included lifestyle modifications (diet, exercise, weight loss, reducing alcohol/caffeine consumption, etc.), oral devices worn during sleep to prevent blocked airways and/or positive airway pressure ventilation. Notably, on December 20, 2024, the FDA approved tirzepatide (Zepbound, Eli Lilly, Indianapolis), a potent GLP-1 agonist, as the first prescription medication for the treatment of moderate-to-severe OSA in adults with obesity.
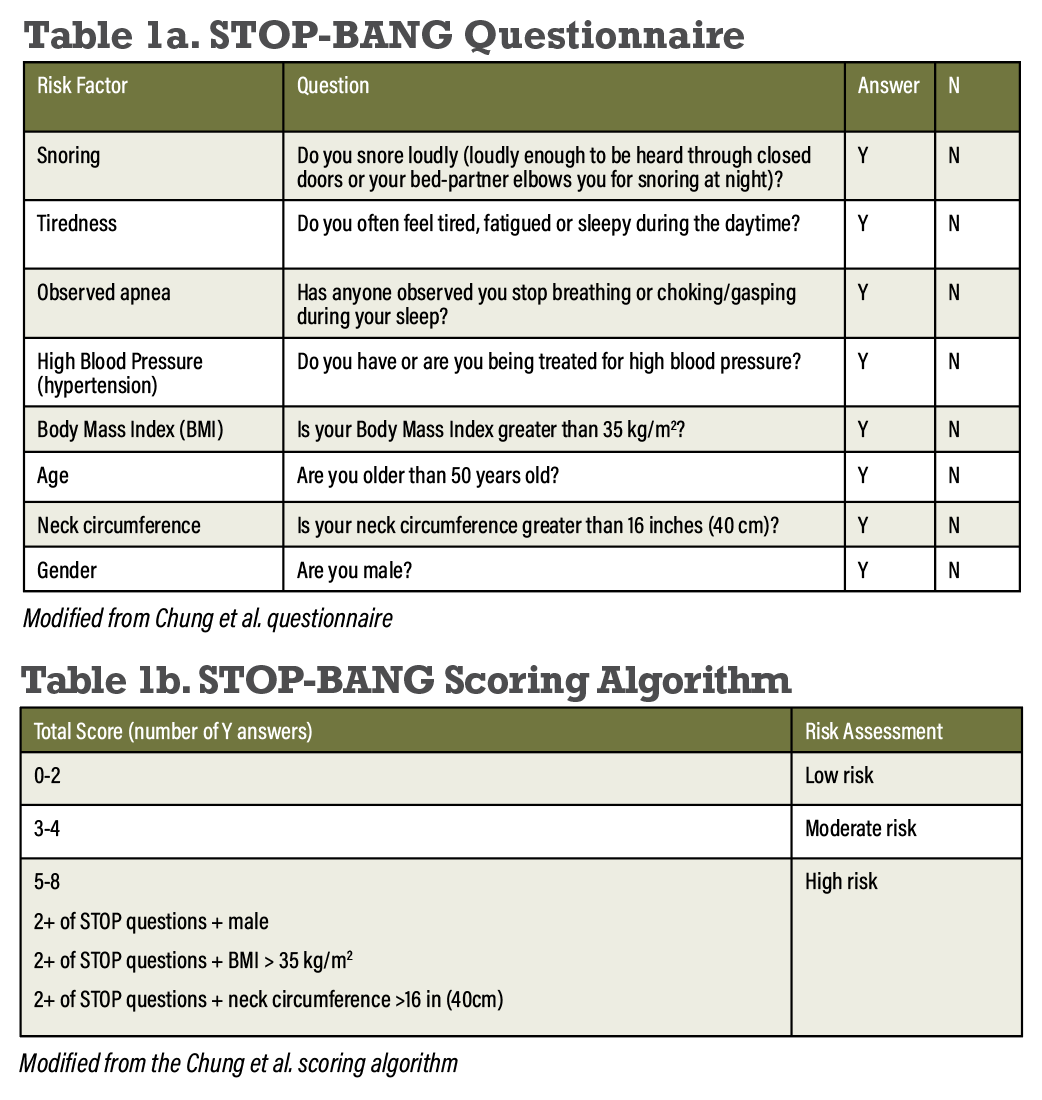
Screening for OSA should include three main strategies: identifying retinal symptoms associated with OSA, such as the presence of cotton wool spots in patients with NPDR; use of the STOP-BANG questionnaire to identify OSA risk factors; and confirming suspected cases of OSA through formal diagnostic polysomnography testing.2 The STOP-BANG questionnaire assesses the eight major risk factors for OSA, which include snoring, tiredness, observed apnea, high blood pressure, body mass index, age, neck circumference and gender (Tables 1a and 1b).14
Other studies have demonstrated that management with continuous positive airway pressure can result in more optimal DR outcomes. One study concluded that CPAP use enhanced response to VEGF therapy, with patients requiring fewer injections of Avastin (bevacizumab) compared to individuals with untreated OSA.15 This introduces a potential gap in care for DR if patients aren’t properly screened for OSA and the condition isn’t addressed. Furthermore, OSA is associated with an increased risk of systemic vascular events, including death, underscoring its broader impact on patient health.2 Implementing the STOP-BANG questionnaire in eye clinics could help identify high-risk patients, ultimately improving both ocular and overall health outcomes.
Due to the nature of the EHR-based study (referenced above) and its reliance on aggregate data, there was the potential for misclassification of DR stages due to inaccuracies in ICD-10 diagnoses and CPT coding. Additionally, the study wasn’t able to account for the severity of OSA or patient compliance with CPAP therapy, thereby limiting observation on how the severity of OSA might affect DR progression and outcomes. It can’t be concluded based on the dataset that was used if compliant management with CPAP positively mitigates the risk of DR progression and to what extent, so this is an area that warrants future interventional studies. Assessing whether CPAP compliance influences DR risk could have significant therapeutic implications. Regardless, there were still observed differences in the rate of progression to more advanced stages and the need for intervention by ophthalmologists.
While complex, the relationship between OSA and DR has been demonstrated to be significant both in the rate of DR progression locally, as well as for systemic morbidity and mortality of afflicted patients. Given that OSA is widely underdiagnosed despite its high prevalence and potential severity, there’s a need to better understand the nature of OSA and its impact on DR in patients so that ophthalmologists can be more proactive in managing these patients and referring them for an appropriate higher level of care.
Bottom line
Ultimately, OSA has been demonstrated to be a risk factor for the progression of DR to more severe stages, PDR and DME, and a greater risk of visual impairment as well as increased treatment burden. Earlier recognition of OSA and referral for treatment offer an opportunity to improve outcomes through proper screening and management, potentially mitigating their DR progression as well as their risk of systemic complications. Further research is needed to better understand the complex nature of the association between OSA and DR. RS
REFERENCES
1. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17.
2. Rahimy E, Koo EB, Wai KM, et al. Impact of obstructive sleep apnea on diabetic retinopathy progression and systemic complications. Am J Ophthalmol. 2025;270:93-102.
3. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:13:1227-1239.
4. Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:2:144-153.
5. Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:6:1017-1019.
6. Chou KT, Huang CC, Tsai DC, et al. Sleep apnea and risk of retinal vein occlusion: A nationwide population-based study of Taiwanese. Am J Ophthalmol. 2012;154:1:200-205.e1.
7. Chatziralli I, Kabanarou SA, Parikakis E, et al. Risk factors for central serous chorioretinopathy: Multivariate approach in a case-control study. Curr Eye Res. 2017;42:7:1069-1073.
8. D’Souza H, Kapoor KG. Retinal vascular manifestations of obstructive sleep apnea. Curr Opin Ophthalmol. 2020;31:6:508.
9. Chang AC, Fox TP, Wang S, Wu AY. Relationship between obstructive sleep apnea and the presence and severity of diabetic retinopathy. Retina (Phila Pa). 2018;38:11:2197-2206.
10. Tan CX, Gao Y, Wang C, et al. [Clinical characteristics of patients with type 2 diabetes mellitus and obstructive sleep apnea syndrome]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:3:441-445.
11. Schober AK, Neurath MF, Harsch IA. Prevalence of sleep apnoea in diabetic patients. Clin Respir J. 2011;5:3:165-172.
12. Al Saeed AA, AlShabib NS, Al Taisan AA, Kreary YA. Association of retinal vascular manifestation and obstructive sleep apnea (OSA): a narrative review. Clin Ophthalmol. 2021;15:3315-3320.
13. Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: Findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:3:702-709.
14. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: A practical approach to screen forOSA. Chest. 2016;149:3:631.
15. Schaal S, Sherman MP, Nesmith B, Barak Y. Untreated obstructive sleep apnea hinders response to bevacizumab in age-related macular degeneration. Retina. 2016;36:4:791-797.



