Take-home points
|
 |
Bios Dr. Popovic is a medical retina fellow at the Doheny and Stein Eye Institutes, University of California Los Angeles. Dr. Ip holds the Gavin S. Herbert Endowed Chair for Macular Degeneration at Doheny Eye Centers-UCLA and is the chief of the Vitreoretinal Surgery Service at the Doheny Eye Centers, UCLA. |
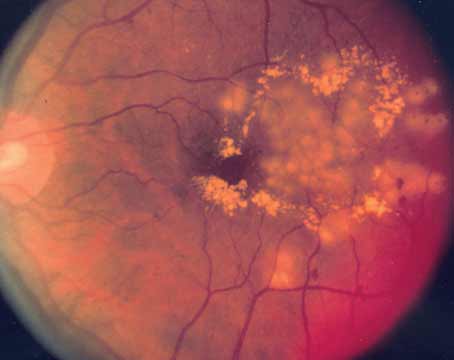
Macular edema secondary to retinal vein occlusion is a common cause of vision loss in this patient population. While traditional anti-vascular endothelial growth factor therapies have revolutionized the care of patients with RVO, treatment responses are variable, and extended durability of treatment is often needed. As treatment paradigms for this condition continue to evolve, here we aim to summarize promising therapeutic advances in patients with RVO.
Faricimab
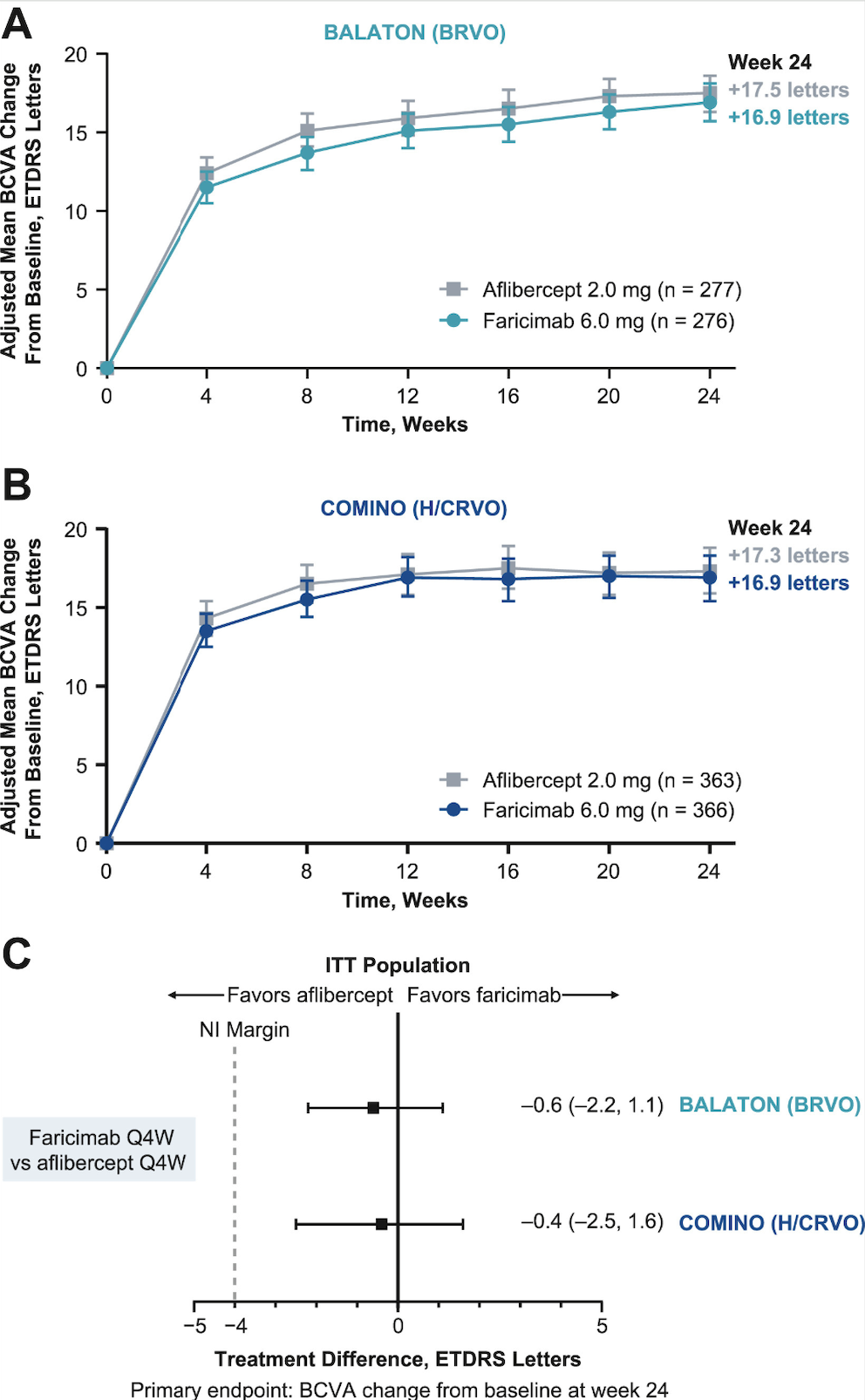
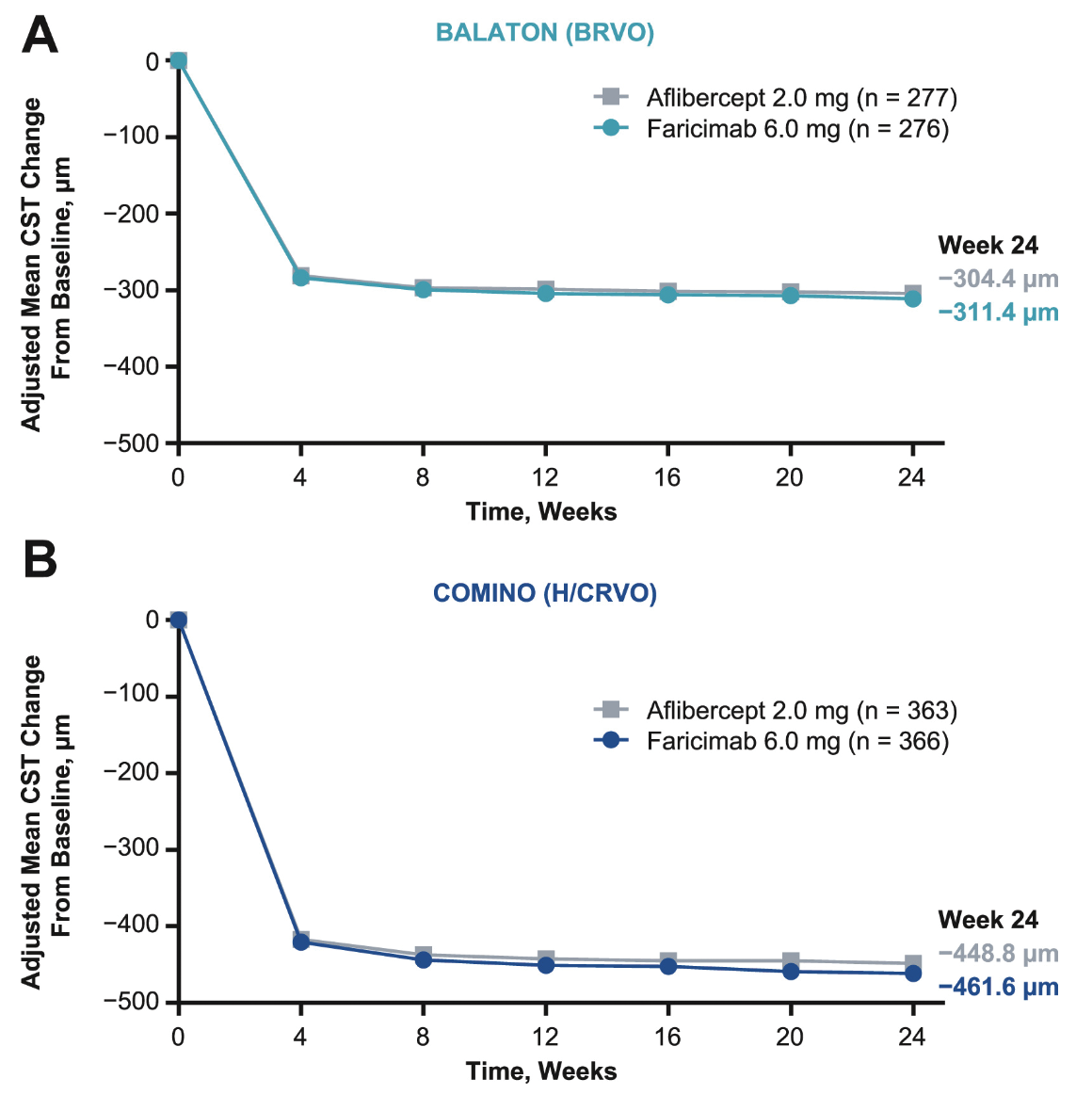
Faricimab is a novel bispecific anti-VEGF and anti-angiopoietin-2 antibody which has already been approved by the FDA for macular edema secondary to RVO and is in clinical use. To support approval of faricimab in this setting, the Phase III BALATON and COMINO trials randomized patients 1:1 to faricimab-svoa 6 mg or aflibercept 2 mg every four weeks for 24 weeks for patients with macular edema secondary to RVO.1 BALATON (n=553) included patients with branch RVO, while COMINO (n=729) considered patients with central or hemiretinal RVO. When considering the primary endpoint of best-corrected visual acuity change from baseline to week 24, faricimab-svoa was noninferior to aflibercept, both in BALATON (adjusted mean change: +16.9 ETDRS letters (95% confidence interval [CI]: 15.7-18.1) vs +17.5 letters (95%CI: 16.3-18.6) and COMINO (+16.9 letters [95%CI: 15.4-18.3] vs +17.3 letters [95%CI: 15.9-18.8]). There were comparable adjusted reductions in mean central subfield thickness (CST) between faricimab and aflibercept by week 24 in both trials. When absence of macular leakage on intravenous fluorescein angiography was considered, there was a greater proportion of patients achieving this endpoint with faricimab 6 mg compared to aflibercept 2 mg in both BALATON (33.6 percent vs 21 percent) and COMINO (44.4 percent vs 30 percent). The ocular adverse events were also comparable between faricimab and aflibercept. Based on these results, the FDA approved Vabysmo (faricimab-svoa) for the treatment of retinal vein occlusion in October 2023.2 Unpublished data from Roche have recently noted updates on the efficacy and safety of faricimab for RVO up to 72 weeks, whereby participants received faricimab in a personalized treat-and-extend dosing regimen based on patients’ response to treatment starting at week 24.3 Some eyes receiving faricimab were able to extend treatment up to every four months, and vision gains and drying of retinal fluid were maintained up to week 72.
 |
|
Graphs showing adjusted mean change in best-corrected visual acuity from baseline over six months in (A) BALATON and (B) COMINO and difference in adjusted mean BCVA change from baseline at the primary end point visits (C). All observed values were used regardless of the occurrence of the intercurrent events. (Source: Tadayoni R, Paris L, Danzig C, et al. Efficacy and safety of faricimab for macular edema due to retinal vein occlusion: 24-Week results from the BALATON and COMINO trials. Ophthalmology 2024;131:8:950-960. Used under Creative Commons License.) |
 |
|
Line graphs showing adjusted mean change in central subfield thickness (CST) from baseline over six months in (A) BALATON and (B) COMINO. (Source: Tadayoni R, Paris L, Danzig C, et al. Efficacy and safety of faricimab for macular edema due to retinal vein occlusion: 24-Week results from the BALATON and COMINO trials. Ophthalmology 2024;131:8:950-960. Used under Creative Commons License.) |
Aflibercept
High-dose aflibercept has been FDA-approved for neovascular age-related macular degeneration and diabetic macular edema, however not for RVO. Approvals for neovascular AMD and DME were based on 48-week data from the PULSAR and PHOTON Phase III randomized trials, respectively, where patients demonstrated clinical equivalent vision gains following either 12- or 16- week dosing regimens, compared with an eight-week dosing regimen for standard dose aflibercept, both after three initial monthly doses.4,5 The randomized Phase III QUASAR study is currently underway to explore the efficacy and safety of aflibercept 8 mg in patients with macular edema secondary to RVO, and the study expects to enroll approximately 800 patients in 27 countries.6
Our understanding of the treatment of macular edema from RVO with standard of care anti-VEGF agents continues to advance. The COPERNICUS and GALILEO Phase III trials randomized patients to sham injections compared to intravitreal aflibercept 2 mg every four weeks for 24 weeks. These trials found a significantly greater BCVA improvement and reduction in central retinal thickness with patients treated with aflibercept, which led to the original approval for aflibercept 2 mg in patients with RVO.7 More recently, the extent of exposure to residual intraretinal fluid (IRF) and fluctuation in central subfield thickness (CST) was correlated to visual outcomes in patients who received aflibercept 2 mg in these two trials.8 Patients were stratified into one of three groups based on the number of visits with IRF, and CST fluctuations were evaluated based on quartiles or tertiles of CST standard deviation.
At week 24, eyes with the greatest number of visits with IRF had poorer BCVA gains from baseline compared to those with the least IRF exposure across a combined dataset of both trials (least-square mean difference: -5.9 letters, [95%CI: -10.0, -1.7]). Additionally, eyes with the highest fluctuation in CST had relatively worse BCVA gains from baseline compared to those with the lowest CST fluctuation (least-square mean difference: -4.6 letters, [95%CI: -9.3, 0.1]). These findings suggest that one of the unmet needs in the treatment of RVO is reducing the persistence of IRF and minimizing CST fluctuations.
The Port Delivery System
Beyond traditional intravitreal anti-VEGF therapies, the port delivery system (PDS) with ranibizumab (Susvimo) represents a novel delivery mechanism designed for continuous delivery of ranibizumab into the vitreous to maintain constant levels of the drug over time. The PDS was evaluated in the Phase III ARCHWAY trial (n=415 patients), which randomized patients with neovascular AMD to ranibizumab PDS implantation with refill-exchanges every 24 weeks compared to monthly ranibizumab.9 In this trial, PDS was found to be noninferior to monthly ranibizumab based on differences in adjusted mean BCVA change from baseline at two years, with approximately 95 percent of patients receiving PDS not requiring supplemental ranibizumab treatment. While cataract was the most common adverse event encountered (8.9 percent following PDS and 6 percent following monthly ranibizumab), other adverse events following PDS included conjunctival erosions (4 percent), conjunctival retractions (2.4 percent), endophthalmitis (1.6 percent) and implant dislocations (1.6 percent). While the PDS implant was FDA approved in 2021, it was voluntarily recalled by Genentech in 2022 due to an investigation related to septum dislodgement cases during the Phase III clinical trial. In July 2024, the PDS was reintroduced following component-level updates to the PDS implant and refill needle.10 As of September 2024, the PDS is currently being evaluated in Phase III trials for DME, as well as diabetic retinopathy without DME, however there are no current trials underway for RVO.11
Gene Therapy
Gene therapies also represent a potential promising future treatment modality in RVO. Gene therapies in this setting aim to be minimally invasive, one-time treatments whereby an adenovirus vector is used to deliver the anti-VEGF gene.12
Ixoberogene soroparvovec (ixo-vec) is a AAV-7m8 vector which encodes aflibercept, and is currently in a Phase II clinical trial aimed at evaluation of efficacy and safety in patients with neovascular AMD. In a Phase I, open label trial, 30 patients with neovascular AMD received an in-office ixo-vec therapy at one of four different doses, and it was found that the therapy was well tolerated with maintenance of vision and improvement of anatomical outcomes.13
RGX-314 is an adeno-associated virus serotype 8 vector which expresses a monoclonal antibody fragment similar to ranibizumab.12,14 In a Phase I/IIa open-label, dose-escalation study, 42 patients with neovascular AMD who were previously treated with anti-VEGF injections received a single subretinal RGX-314 injection by a trained vitreoretinal surgeon.14 There was one serious adverse event possibly related to RGX-314, in which a patient developed severe vision loss due to pigmentary changes in the macula; additionally, asymptomatic pigmentary changes in the inferior retinal periphery were seen in some patients who received RGX-314. Doses of 6 x 1010 genome copies or higher were generally associated with stable or improved BCVA and CST, with most participants needing few or no supplemental anti-VEGF injections.
To date, ixoberogene soroparvovec and RGX-314 have been primarily tested in neovascular AMD patients, however their future application to RVO deserves consideration.
Steroid Implants
Though it hasn’t been the subject of a formal drug trial, physicians have used the fluocinolone acetonide intravitreal implant (Iluvien) off-label in cases of RVO in an effort to decrease the treatment burden.
In one case report, a patient with non-ischemic CRVO was initially treated with injections of dexamethasone. However, researchers reported that these caused fluctuations in vision between 20/32 and 20/200 due to the presence of macular edema. When the FA implant was used, however, the researchers reported “sustained improvement in visual acuity from 20/200 to 20/25.”15
The FA implant has also been used off-label in cases of ischemic CRVO and BRVO. In one case, a patient with CRVO presented with vision of 29 letters and a central retinal thickness of 664 µm.16 After showing no response to anti-VEGF and triamcinolone injections, and just a temporary anatomical improvement after several dexamethasone implants (Ozurdex), she was implanted with the FA implant. This resulted in “significant and sustained” vision (BCVA of 38 letters) and anatomical improvement (CRT of 271 µm). The researchers reported similar improvements in a BRVO patient.16
Bottom Line
Significant attention and interest has been garnered for newer “second generation” treatment modalities in patients with RVO. With respect to anti-VEGF therapies, faricimab has already received FDA approval in RVO, while aflibercept 8 mg is currently undergoing a Phase III trial for this condition at sites internationally. The port delivery system of ranibizumab and gene therapies have been primarily evaluated in the setting of neovascular AMD; however, these treatments and the fluocinolone implant also carry promise for patients with RVO. Our understanding of novel treatment strategies in RVO continues to evolve, and together, these advances pave the way for improved outcomes in patients with this sight-threatening condition. RS
REFERENCES
1. Tadayoni R, Paris LP, Danzig CJ et al. Efficacy and safety of faricimab for macular edema due to retinal vein occlusion: 24-week results from the BALATON and COMINO trials. Ophthalmology 2024;131:8:P950-960.
2. Genentech. FDA approves Genentech’s Vabysmo for the treatment of retinal vein occlusion (RVO). Press Release: October 2023. Available at: https://www.gene.com/media/press-releases/15009/2023-10-26/fda-approves-genentechs-vabysmo-for-the.
3. F. Hoffman-La Roche. Roche’s Vabysmo maintained vision improvements with extended treatment intervals up to four months for people with retinal vein occlusion (RVO) in phase III studies. Press Release: October 2023. Available at: https://www.globenewswire.com/news-release/2023/10/10/2757105/0/en/Roche-s-Vabysmo-maintained-vision-improvements-with-extended-treatment-intervals-up-to-four-months-for-people-with-retinal-vein-occlusion-RVO-in-phase-III-studies.html.
4. Brown DM, Boyer DS, Do DV et al. Intravitreal aflibercept 8 mg in diabetic macular oedema (PHOTON): 48-week results from a randomised, double-masked, non-inferiority, phase 2/3 trial. Lancet 2024;403:10432:1153-1163.
5. Lanzetta P, Korobelnik JF, Heier JS et al. Intravitreal aflibercept 8mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet 2024;403:10432:1141-1152.
6. Schulze J. Bayer initiates phase III study to investigate aflibercept 8 mg in retinal vein occlusion. Press Release: May 2023. Available at: https://www.bayer.com/media/en-us/bayer-initiates-phase-iii-study-to-investigate-aflibercept-8-mg-in-retinal-vein-occlusion/.
7. Pielen A, Clark WL, Boyer DS et al. Integrated results from the COPERNICUS and GALILEO studies. Clin Ophthalmol 2017;11:1533-1540.
8. Ip MS. Impact of exposure to residual intraretinal fluid and fluctuations of central subfield thickness on visual outcomes in eyes with macular edema following central retinal vein occlusion: a 1-year post hoc analysis of the COPERNICUS and GALILEO trials. Invest Ophthalmol Vis Sci 2024;65:780.
9. Regillo C, Berger B, Brooks L et al. Archway phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration 2-year results. Ophthalmology 2023;130:7:735-747.
10. Genentech. Genentech to reintroduce Susvimo for people with wet age-related macular degeneration (AMD). Press Release: July 2024. Available at: https://www.gene.com/media/press-releases/15031/2024-07-08/genentech-to-reintroduce-susvimo-for-peo.
11. Genentech. Pipeline. September 2024. Available at: https://www.gene.com/medical-professionals/pipeline.
12. Lendzioszek M, Bryl A, Poppe E et al. Retinal vein occlusion-background knowledge and foreground knowledge prospects-a review. J Clin Med 2024;13:13:3950.
13. Khanani AM, Boyer DS, Wykoff CC et al. Safety and efficacy of ixoberogene soroparvovec in neovascular age-related macular degeneration in the United States (OPTIC): A prospective, two-year, multicentre phase 1 study. eClinicalMedicine 2024;67:102394.
14. Campochiaro PA, Avery R, Brown DM et al. Gene therapy for neovascular age-related macular degeneration by subretinal delivery of RGX-314: A phase 1/2a dose-escalation study. Lancet 2024;403:10436:1563-1573.
15. Coelho J, Pessoa B, Meireles A. Long-term management of non-ischemic central retinal vein occlusion with fluocinolone acetonide intravitreal implant 190 mug (ILUVIEN). Ther Adv Ophthalmol 2019;11:2515841418820755.
16. Ribeiro M, Pereira AF, Penas S, Falcão-Reis F, Beato JN. 12-month effectiveness of the fluocinolone acetonide implant (Iluvien) in retinal vein occlusions – Two clinical cases. EURETINA 2021 (online meeting).