Take-home points
|
 |
Bios Dr. Thangamathesvaran is a vitreoretinal surgery fellow at Wilmer Eye Institute, Johns Hopkins University Hospital, Baltimore. Dr. Arevalo is the Edmund F. and Virginia B. Ball Professor of Ophthalmology in the retina division of Wilmer Eye Institute and chairman of ophthalmology at Johns Hopkins Bayview Medical Center. DISCLOSURES: The authors have no financial relationships to disclose. |
Full-thickness macular holes are complete defects in the neurosensory retina that are a result of multiple etiologies, including, but not limited to, vitreomacular traction, trauma, retinal detachment or uveitis. The pathophysiology of the most common etiology, VMT, includes a tractional force resulting in localized thinning of the retina that causes a progressive defect with complete loss of retinal tissue.1
Conventionally, the most definitive management of full-thickness macular hole (FTMH) has been pars plana vitrectomy with or without internal limiting membrane peel and tamponade with gas or silicone oil. However, these interventions present challenges, including complications such as cataract, risk of retinal detachment and the need for face-down positioning.
Although spontaneous hole closure has traditionally been observed with traumatic macular holes, with closure rates ranging from 10 to 50 percent, and reports recommending observation for up to four months to see if traumatic holes close, newer studies are finding spontaneous closures with observation and nonsurgical interventions in non-traumatic FTMH.2
This article will review anatomical characteristics of holes that are more likely to spontaneously close, variations in the noninterventional management options, and how to predict which holes will need surgery once these interventions are put in place.
Imaging markers of spontaneous FTMH
Our group studied imaging features associated with closure of FTMH.3 The retrospective study included cases with an established diagnosis of FTMH, as determined by spectral-domain optical coherence tomography, that were followed without surgery and collected from retina specialists worldwide. Successful closure was defined as flattening and reattachment of the hole rim along the entire circumference of the hole.
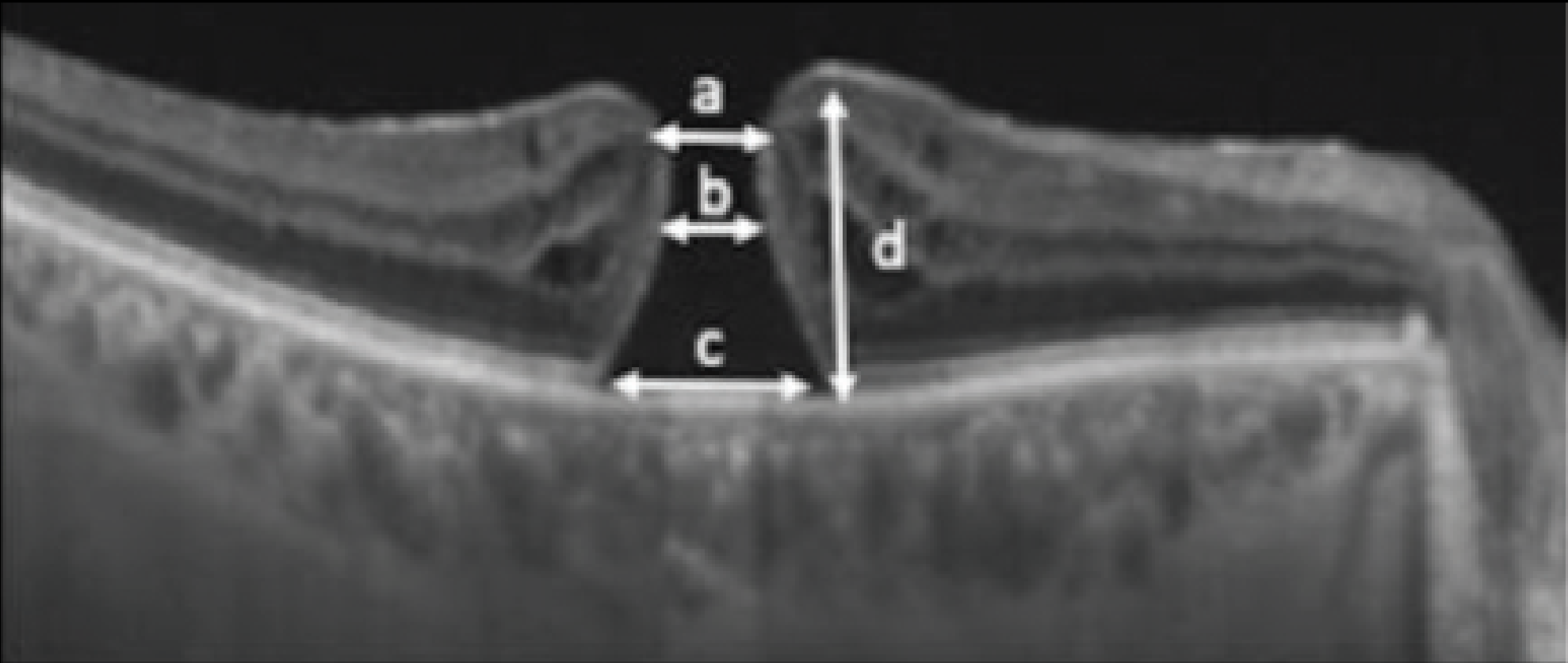
Exclusion criteria included presence of retinal dystrophy, foveoschisis, neovascular age-related macular degeneration and history of vitreous surgery less than one year prior. SD-OCT scans were reviewed for macular parameters including apical diameter, narrowest diameter, basal diameter and height (Figure 1).
 |
|
Figure 1. A diagram of full-thickness macular hole parameters: a = apical diameter; b = minimum diameter; c = basal diameter; and d = height. (Reprinted with permission from Uwaydat SH, Mansour A, Ascaso FJ, et al. Clinical characteristics of full thickness macular holes that closed without surgery. Br J Ophthalmol. 2022;106:1463–1468.)3 |
Additional FTMH parameters that we evaluated include macular hole index (height/basal diameter), diameter hole index (DHI, an indicator of tangential traction defined as narrowest diameter divided by basal diameter), tractional hole index (THI, an indicator of anteroposterior traction and retinal hydration defined as height/narrowest diameter) and subretinal fluid volume.
Researchers in Spain previously evaluated these markers as potential predictors of successful outcomes in FTMH surgery. They identified a narrowest diameter for smaller macular hole and a larger tractional hole index as predictive factors for a good visual prognosis after surgery.4
SD-OCT parameters that characterized the vitreoretinal interface included the presence of vitreomacular traction in 12 (15.8 percent) eyes, perifoveal PVD in 42 (53.8 percent), foveal epiretinal membrane in 10 (12.8 percent), cystoid macular edema in 49 (62.8 percent) and subretinal fluid in 20 (25.6 percent).
In our study, the FTMH closed in 74 eyes of 78 patients. On multivariate analysis, initial visual acuity correlated to the height and narrowest diameter of the hole while final visual acuity correlated with the basal diameter. Time for closure of FTMH was a median of 2.8 months and correlated to the narrowest diameter and the presence of subretinal fluid. THI negatively correlated with time to closure.
Other studies of spontaneous closure
Other studies that examined characteristics of spontaneous closure found an association of faster closure rates when CME was present because the edema helps reapproximate the hole edges by a mechanism of primary intention.5,6 This might play an important role in the management of FTMH associated with uveitis.
A study this year by Jessie Wang, MD, and colleagues, identified factors associated with decreased rates of spontaneous closure, which included increasing hole size, as every 10 µm decrease in size correlated to an increase in closure by a factor of 1.2.7 The study also highlighted the presence of VMT related inversely to successful closure.
The study further corroborated this with the finding that patients who had undergone vitrectomy had higher closure rates due to the absence of tractional forces. Furthermore, the study found that the best-corrected visual acuity was better in patients whose holes closed with only topical drops compared to holes that required PPV. However, the holes that underwent PPV were significantly larger and had persistent VMT, which could also affect final visual acuity. However, there were no differences in BCVA between patients that underwent surgery after drops vs. surgery at the initial presentation. 7
Agents used for FTMH closure
In our retrospective observational series, 18 eyes received the following nonsurgical interventions, with some eyes receiving multiple interventions: intravitreal anti-VEGF agents (four eyes); topical corticosteroids (13); and topical nonsteroidal anti-inflammatory drugs (seven). We didn’t compare these modalities.
Other studies have used a combination of these modalities, with Dr. Wang’s group using this standardized regimen:
- topical steroids prednisolone acetate 1% every six hours or difluprednate 0.05% every six to 12 hours;
- NSAIDs ketorolac tromethamine 0.5% or bromfenac 0.07% every six to eight hours; and
- carbonic anhydrase inhibitors (CAIs) brinzolamide 1% or dorzolamide 2% every eight to 12 hours.7
In a multicenter U.S. study, participants were started on the following regimen:
- off-label topical prednisolone acetate 1% every six to eight hours or difluprednate 0.05% every eight to 12 hours;
- ketorolac-tromethamine 0.5% every six to eight hours or bromfenac 0.07% every six to 24 hours; and
- brinzolamide 1% or dorzolamide 2% every eight to 12 hours.5
No studies have yet compared the efficacy of these different drop regiments.
Dynamic predictors of FTMH closure
In our study, the mean time to closure from initial detection was 6.2 months with a mean logMAR initial (± standard deviation [SD]) visual acuity improved from 0.65 ±0.54 to 0.34 ±0.45 (Snellen equivalent 20/89 to 20/44). The initially closed FTMH reopened in seven eyes (9 percent) after a mean of 8.6 months.
Among the eyes that reopened, two closed and one stayed open after vitrectomy, and one closed on topical steroids and topical NSAIDs. Four eyes had no surgery. A total of 74 eyes had stable or improved final vision, while four eyes lost vision due to a subfoveal scar after blunt trauma (two eyes) and foveal RPE atrophy (two eyes). Three FTMH resolved after VMT resolution and three closed after a new occurrence of PVD. One FTMH closed despite the persistence of VMT.
Mean time for closure was:
- 1.6 months for eyes with trauma;
- 4.3 months for eyes without trauma but that had therapy for CME;
- 4.4 months for eyes without trauma and without therapy if the holes were <200 µm in size; and
- 24.7 months for holes >200 µm.
Figure 2 shows a case of a patient that had sustained a sports-related injury and started on topical NSAIDs, with hole closure resulting in visual acuity improvement from 20/40 to 20/20.
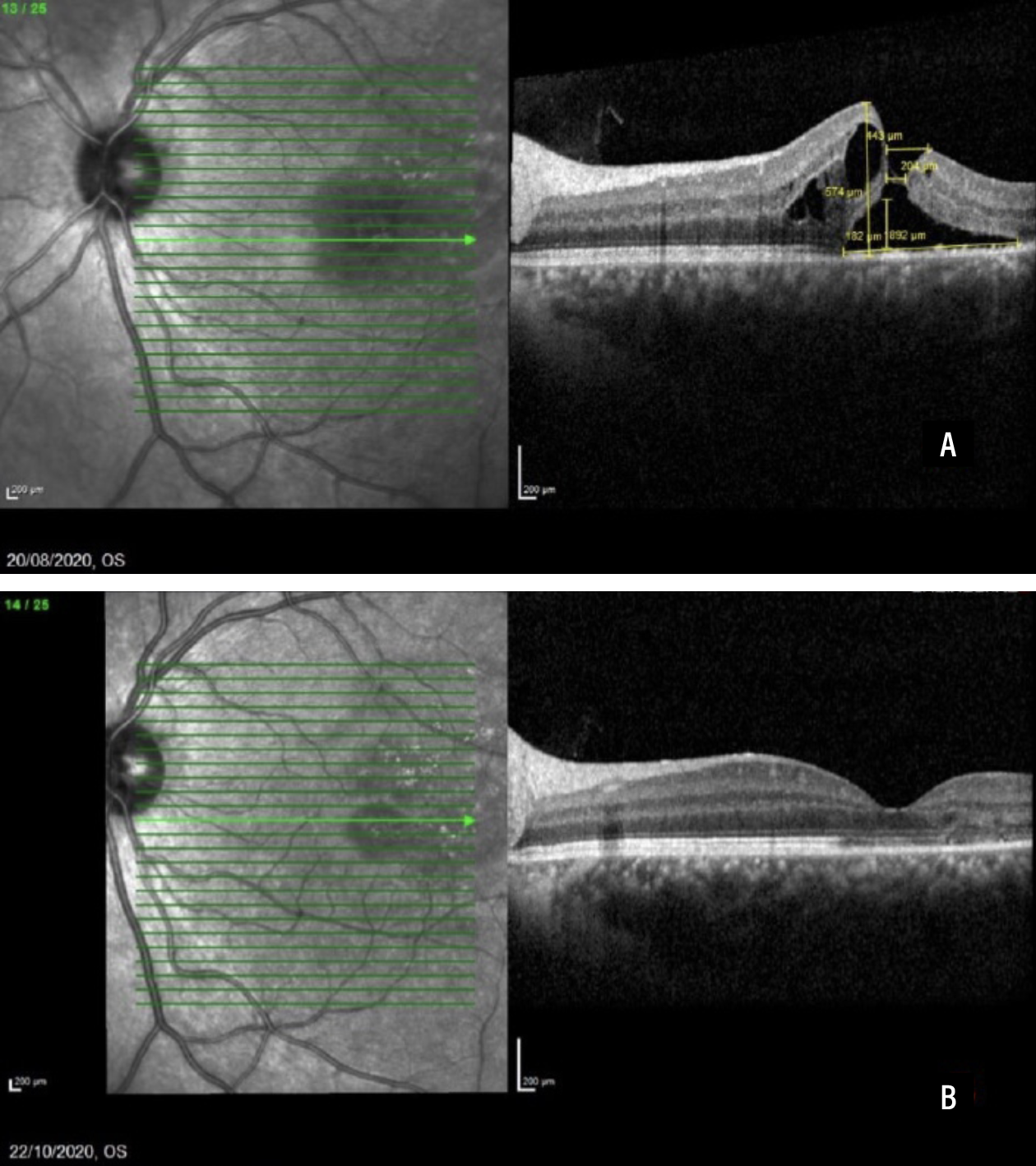 |
| Figure 2. Spectral-domain optical coherence scans before full-thickness macular hole closure (A) and two months after closure (B). This 24-year-old White male sustained a sports injury and was started on topical NSAID. Visual acuity improved from 6/12 (20/40) to 6/6 (20/20). (Reprinted with permission from Uwaydat SH, Mansour A, Ascaso FJ, et al. Clinical characteristics of full thickness macular holes that closed without surgery. Br J Ophthalmol. 2022;106:1463–1468.)3 |
Dr. Wang’s group noticed that when tracking the progression of FTMH size over time, the eyes that responded to drop therapy showed a rapid reduction in size. In particular, rates of macular hole narrowing and reduction in central foveal thickness acted as indicators for drop effectiveness.7 In total 36.7 percent of patients achieved hole closure; however, final visual acuity didn’t differ between eyes undergoing primary PPV vs. those taking drops before undergoing PPV. The study didn’t report on reopening of FTMH.
In the multicenter U.S. study, OCT studies showed decreased cystoid changes within two to four weeks and FTMH closure with visual acuity improvement in two to eight weeks in most eyes. The median treatment duration until initial closure was approximately 5.6 weeks. Total duration of drop treatment, including taper, ranged from 3.5 to 20 months. Two patients had FTMH recurrence at six months after an initially successful closure, and two others subsequently needed PPV for visually significant epiretinal membrane.
Anatomical markers
 |
These studies highlight the anatomical characteristics that are important when considering which FTMH are more likely to spontaneously close. First, FTMH <200 µm, especially in the setting of trauma and without the presence of VMT, have a high likelihood of closure.8 In addition, the absence of VMT is important because persistent tractional forces can overwhelm the RPE pump, preventing hole closure.
These studies also highlight that the use of drops can help improve the closure process without affecting the final outcomes. When comparing closure rates in patients who had FTMH closure vs. those who didn’t, the participants who used drops had greater closure rates.8,9
Furthermore, in participants who had successful hole closure rates, there was a significant reduction in macular hole size and changes in SD-OCT markers early on in the treatment, highlighting the importance of close monitoring and quick intervention if there’s no response. In particular, Dr. Wang’s group recommended scheduling a tentative date for surgery even in the group that initiates drop therapy to prevent any surgical delays. Finally, it’s important to review the adverse effects of these topical agents, including corneal melt, ocular surface irritation and elevated intraocular pressure.
Future studies
We need prospective, randomized, double-masked, high-powered studies in the future to improve our clinical management and to help us apply these principles into practice. First, studies that compare different noninterventional therapeutic protocols will be helpful to determine whether individual agents or specific combinations will help promote FTMH closure. This is particularly useful in patients who have contraindications to certain agents, such as topical corticosteroids (in patients with glaucoma) and topical NSAIDs (in patients with corneal surface disease).
Second, knowing how systemic conditions, such as diabetes, affect rate of hole closure can help clinical decision-making. Finally, as artificial intelligence continues to shape how we practice medicine, it will be useful to identify if OCT biomarkers can be put into an algorithm to help predict which patients can be observed and who would need surgery, and then use the dynamic response from their OCT scans to further refine clinical decision-making.
Bottom Line
Not all FTMH require surgery. Careful analysis of SD-OCT markers can help determine which FTMH might close on their own, with close vigilance to treatment response being essential in avoiding lapses in surgical intervention. RS
REFERENCES
1. Bikbova G, Oshitari T, Baba T, Yamamoto S, Mori K. Pathogenesis and management of macular hole: review of current advances. J Ophthalmol. 2019;2019:3467381.
2. Yamashita T, Uemara A, Uchino E, Doi N, Ohba N. Spontaneous closure of traumatic macular hole. Am J Ophthalmol. 2002;133:230–235.
3. Uwaydat SH, Mansour A, Ascaso FJ, et al. Clinical characteristics of full thickness macular holes that closed without surgery. Br J Ophthalmol. 2022;106:1463–1468.
4. Ruiz-Moreno JM, Staicu C, Piñero DP, Montero J, Lugo F, Amat P. Optical coherence tomography predictive factors for macular hole surgery outcome. Br J Ophthalmol. 2008;92:640–644.
5. Sokol JT, Schechet SA, Komati R, et al. Macular hole closure with medical treatment. Ophthalmol Retina. 2021;5:711–713.
6. Elhusseiny AM, Smiddy WE, Flynn HW, Schwartz SG. Case series of recurring spontaneous closure of macular hole. Case Rep Ophthalmol Med. 2019;2019:2398342.
7. Wang J, Rodriguez SH, Xiao J, et al. Full-thickness macular hole closure with topical medical therapy. Retina. 2024;44:392–399.
8. Park S, Lim LT, Gavin MP. Topical steroidal and nonsteroidal antiinflammatory drugs for the treatment of cystoid macular edema in retinitis pigmentosa. Retin Cases Brief Rep. 2013;7(2):134–136. doi: 10.1097/ICB.0b013e31825a300f.
9. Sugiyama A, Imasawa M, Chiba T, Iijima H. Reappraisal of spontaneous closure rate of idiopathic full-thickness macular holes. Open Ophthalmol J. 2012;6:73–74.