Take-home points
|
 |
Bios Dr. Bomdica is a vitreoretinal surgery fellow at The Retina Institute in St. Louis. Dr. Blinder is a partner of The Retina Institute in St. Louis, professor of Clinical Ophthalmology and Visual Sciences at Washington University in St. Louis; chair of The Retina Research Committee and co-director of the TRI Retina Fellowship program. Disclosures: Kevin J. Blinder— Astellas/Iveric Bio; Bausch & Lomb; Regeneron; AbbVie (Allergan) and Genentech. |
With an aging population in the United States and other countries, the burden of age-related macular degeneration is rising and unique solutions are necessary to address this increasing demand. Making the job tougher is the fact that the multiple office visits required to treat nAMD can be onerous for patients and their families. The advent of home optical coherence tomography might make this situation a little easier for both them and their physicians. Here we’ll take a look at a recently approved home OCT system and how it might alleviate this treatment burden.
The wet AMD landscape
It’s been well-established that the majority of severe vision loss in AMD is attributable to neovascular AMD.1 nAMD is typically monitored by in office optical coherence tomography, which allows for precise and accurate measurements of subretinal fluid and intraretinal fluid, and fundoscopic examination to assess for submacular hemorrhage, which may be missed on OCT.
Currently, nAMD therapies cost the U.S. health-care system approximately $4 billion dollars annually.2 However, despite this cost, visual outcomes for patients in the real world tend to underperform those cited in the pivotal clinical trials, and this is often attributed to undertreatment.3,4 The treat-and-extend regimen is the most commonly used strategy in the United States, and it’s defined by treating nAMD-associated fluid at regular intervals until dry and then extending the interval.
 |
|
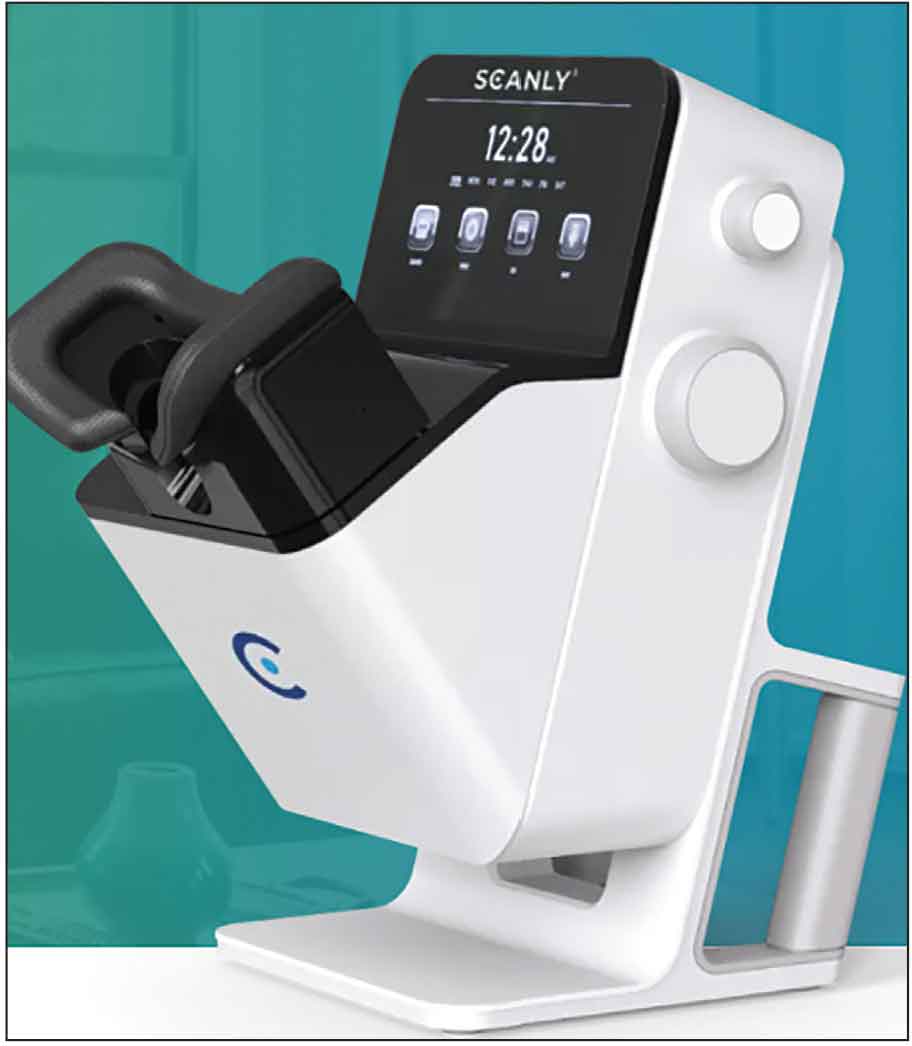
Figure 1. Scanly Home OCT Machine by Notal Vision. |
The significant number of treatments and office visits that nAMD treatment often requires puts a significant burden on these patients. Additionally, nAMD-associated visual impairment can lead to downstream health problems.5 Furthermore, these patients are sometimes unable to transport themselves for a variety of reasons, leading to an additional burden on family members or caregivers. If we could reduce this burden, that would represent a significant quality of life and economic value to patients and/or their caregivers.
One method of reducing this burden is by reducing the number of office visits and treatments. OCT machines have only been conventionally available in clinical settings, but home-OCT machines are now available: The FDA gave breakthrough device designation in 2018 to Notal
Vision Home OCT (NVHO, Notal Vision) device, which uses an AI algorithm called Notal OCT Analyzer (NOA) to measure SRF and IRF daily.6 The device, called the Scanly Home OCT, was subsequently approved by the FDA in May 2024.
The Scanly machine is a compact home-OCT device based on spectral domain-OCT. Each scan is composed of a horizontal raster of 88 B-scans across a 3 x 3 mm area. This data is then transmitted to the HIPAA-compliant Notal Health Cloud portal using either a smartphone or Wi-Fi connection. Cube scans along with analysis of fluid burden (IRF and/or SRF) are then recreated for physician viewing. Manufacturer signal quality index (MSI) is a quantification of the image signal quality, which may factor into a physician’s assessment of the images and fluid quantification. The goal of this machine is to allow for personalized monitoring of each patient and ensure that only those who need treatments travel to a clinical setting. A time-based and fluid-based threshold can be used for easy monitoring of each patient.
 |
|
Figure 2. Online login portal for Home OCT review. |
Home OCT Accuracy and Outcomes
There have been several studies assessing the Scanly/NVHO machine with promising results. Some of these key studies are summarized below.
One group performed a prospective, longitudinal pilot study for the use of NVHO in nAMD patients.6 In particular, they assessed how similar NOA was to manual OCT graders in regard to whether fluid (IRF and/or SRF) was present and fluid volume quantification based on the NVHO scans. They found a high concordance (94.7 percent) between NOA and manual graders in regard to whether fluid was present. Additionally, there was a minimal difference in fluid volume quantification between NOA and human graders (0.3 nL for IRF, 1.2 nL for SRF, and 1.5 nL for total retinal fluid volume).
DRCR Protocol AK was a home OCT feasibility study (n=14 patients), and analyzed concordance between in-office OCT scans and NVHO scans on the same day.7 Thirteen out of 14 participants obtained a scan with good enough quality for analysis by NOA, and the remaining participant did so after 13 days (six attempts). The percentage of acquired scans that were considered “good quality” was 99.3 percent, and the NOA successfully quantified fluid on 86.5 percent of scans. For 35 scan pairs judged as having fluid by in-office OCT, the NOA detected fluid on 31 scans (89 percent). For 14 scan pairs judged as having no fluid on in-office OCT, the NOA didn’t detect fluid on 10 scans (71 percent). For all cases of disagreement, the volume involved was less than 10 µL, which has now become the threshold for the fluid alert.
At the end of the study (approximately six months), survey results revealed that all participants agreed that device instructions were clear, and the daily use of the device was easy and comfortable. Notably, DRCR Protocol AO is currently underway, and aims to assess home OCT-guided treatment compared to a treat-and-extend strategy for nAMD.
A prospective trial compared approximately six months of post-NVHO implementation (n=15 patients, 27 eyes) outcomes to up to two years of retrospective pre-NVHO implementation outcomes within this same cohort.8 NOA quantification of fluid was possible for a mean 91.2 percent of scans. Among the cohort, there was improved speed in OCT acquisition and decreased interquartile range in OCT acquisition time with an increasing number of scans, indicating a positive learning curve over the course of the study. This study also proposed classifications of nAMD fluid trajectories based on home-OCT findings. Importantly, the mean number of weeks before an injection pre-NVHO was 6.2, compared to 11.6 weeks post-NVHO implementation (p=0.0002). Despite the increased interval spacing, there were similar visual acuity changes pre-NVHO compared to post-NVHO implementation (p=0.39).
 |
|
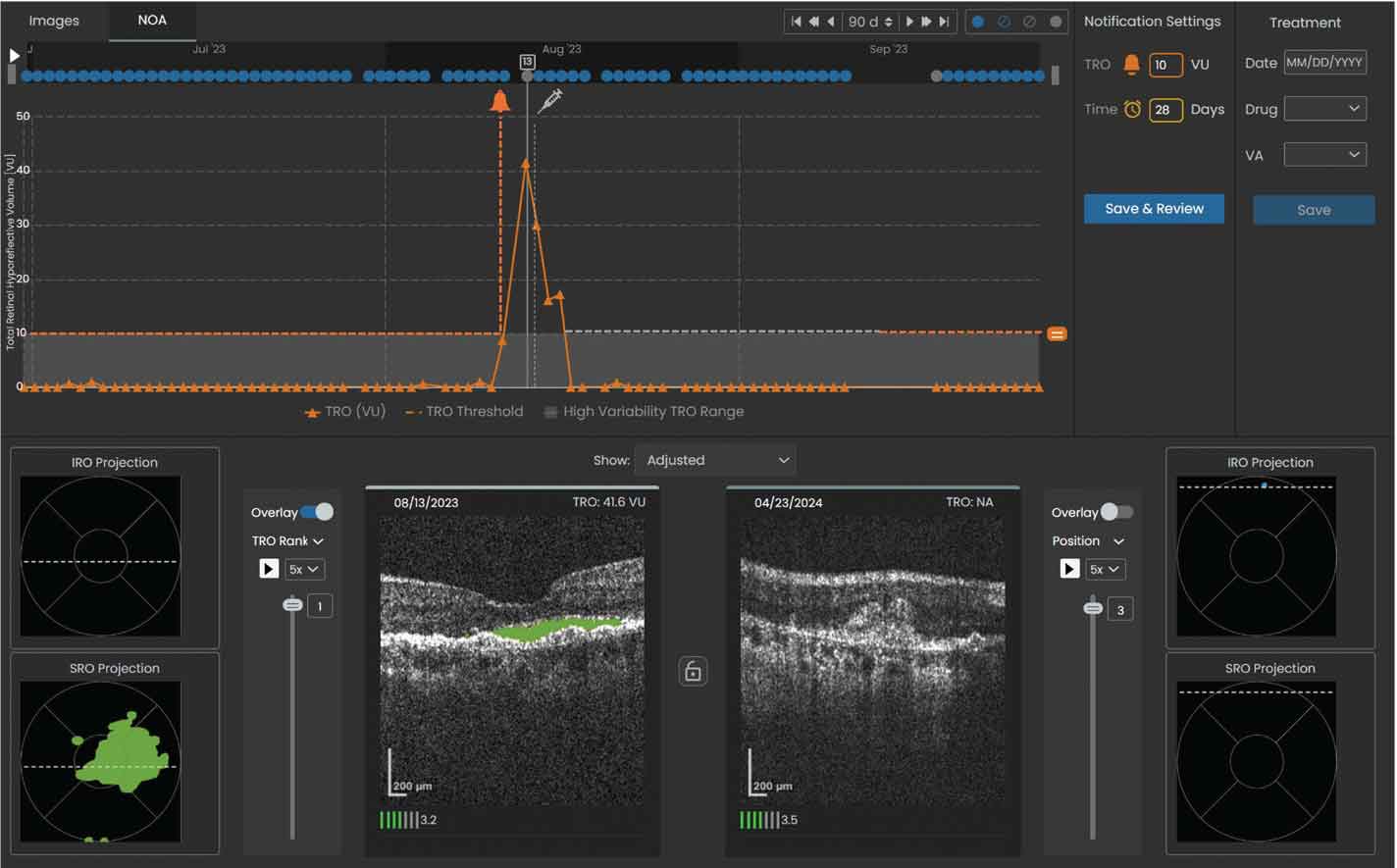
Figure 3. Sample volume trendline showing a notification was sent to the reviewing physician when the total retinal volume crossed the set threshold of 10 µL. The left OCT scan shows the total retinal volume reached a maximum of 41.6 µL within the specified 90-day window on 08/13/2023 and was predominantly SRF (denoted by the shaded green area). |
Integration of Home OCT Into Clinical Practice
1. Identify patients with active nAMD who you feel are cognitively and physically adept enough to use a home OCT machine and who are particularly burdened by office visits and treatments frequency.
2. The home OCT monitoring is provided through an independent diagnostic facility (IDTF), also known as a monitoring center. If the patient is eligible to enroll in the program (based on insurance or patient amenability to paying out of pocket), the retina physician can write a prescription to the monitoring center.
3. The monitoring center will provide the device (Figure 1), facilitate detailed virtual training for the patient, and provide technical and adherence support for the program. Once the patient is trained and set up with the machine, daily scans and fluid analysis can be accessed via the HIPAA-compliant portal (Figure 2).
4. The review of home OCT data can be billed by the physician using dedicated CPT code 0606T for cumulative work every 30 days. The technical component of the home OCT is directly billed by the monitoring center.
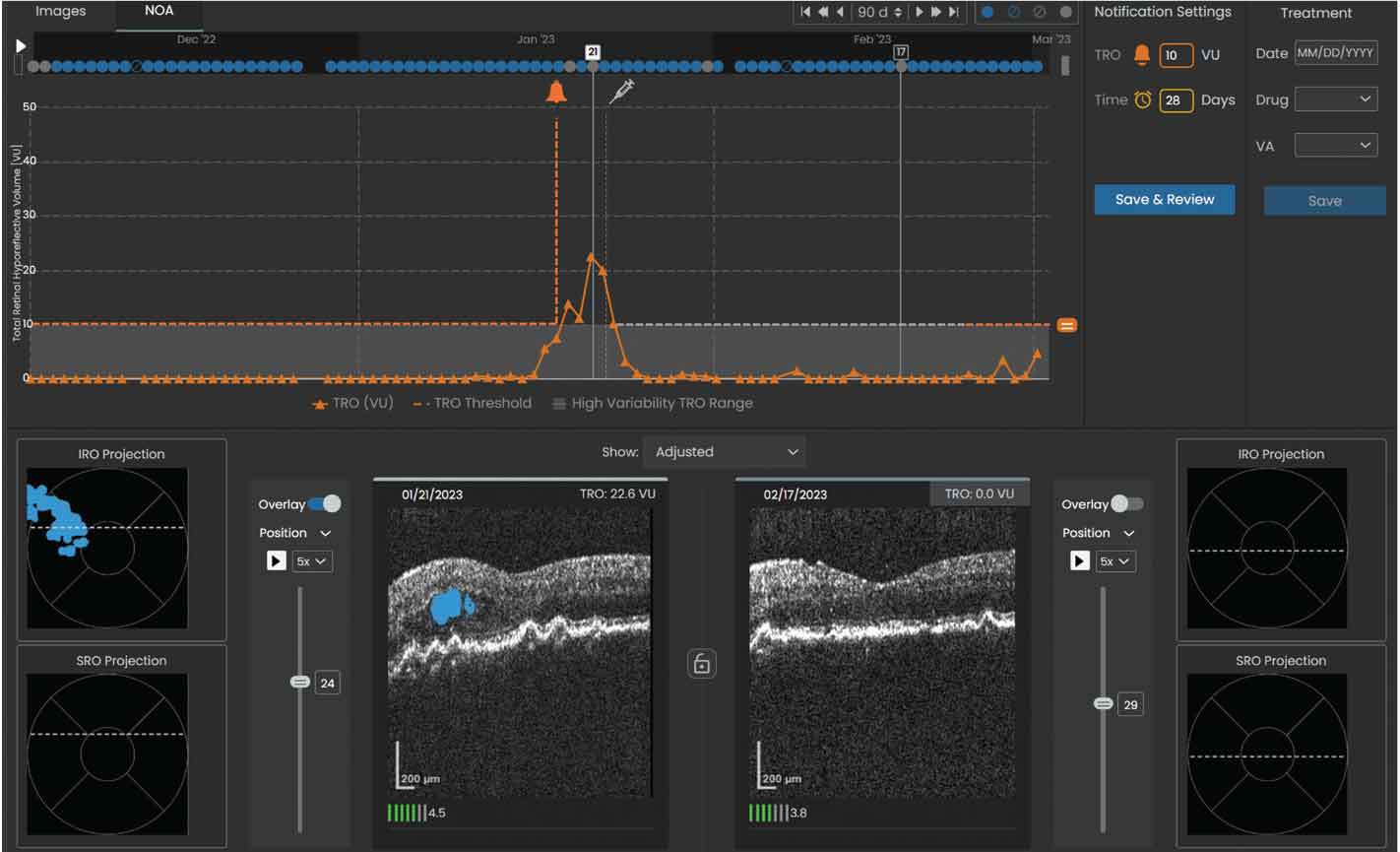
5. If fluid volume or the time interval exceeds the set threshold (Figures 3 and 4), a notification will prompt review by the retina specialist, and the patient may subsequently be scheduled for an in-office evaluation and injection visit.
 |
|
Figure 4. Sample volume trendline showing a notification was sent to the reviewing physician when the total retinal volume crossed the set threshold of 10 µL. The left OCT scan shows the total retinal volume reached a maximum of 22.6 µL within the specified 90-day window on 01/21/23 and was predominantly IRF (denoted by the shaded blue area). |
Limitations
Patients will have to be cognitively and physically able to use home OCT well enough to take good quality scans. Retina specialists will likely prescreen patients based on their own best judgment, and the suitable patients can undergo a standardized training procedure. Furthermore, patients and/or family members or their caregivers must be responsive with regard to any issues and possible treatments on short notice. Although the monitoring center provides a tutorial and machine support for patients, staff within the retina clinic are still necessary to coordinate the short-notice injection visits for these patients, deal with potential billing issues and assist patients with additional questions that may arise.
Currently, the home OCT machine does not measure pigment epithelial detachments. This should be available in upcoming updates. It also doesn’t simultaneously gather macular fundus photography. Therefore, submacular hemorrhages may be missed, although any associated volume change would be detected. As technology evolves, the incorporation of multimodal imaging into a compact form factor may become a reality. Although the current substantiating research studies are promising, they tend to have small sample sizes with short follow-up, so real-world long-term data with a large sample size is yet to be seen. Protocol AO will certainly add to the data set.
Bottom Line
Home OCT technology is still quite new and is being actively researched, but initial results are promising. If scaled in clinical practice, the technology may lead to significantly reduced health-care spending on anti-VEGF agents in the nAMD population (and possibly other macular pathologies in the future) along with improvements in patient quality of life and reduced burden on retina clinics, possibly increasing patients’ access to care for other conditions. RS
REFERENCES
1. Ricci F, Bandello F, Navarra P, Staurenghi G, Stumpp M, Zarbin M. Neovascular age-related macular degeneration: Therapeutic management and new-upcoming approaches. International Journal of Molecular Sciences. 2020;21:21:8242.
2. Patel S, Sternberg P. Is there a cost benefit to the ranibizumab port delivery system? JAMA Ophthalmol. 2022;140:7:723-724.
3. Khanani AM, Skelly A, Bezlyak V, Griner R, Torres LR, Sagkriotis A. SIERRA-AMD: A retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4:2:122-133.
4. Ho AC, Kleinman DM, Lum FC, et al. Baseline visual acuity at wet AMD diagnosis predicts long-term vision outcomes: An analysis of the IRIS Registry. Ophthalmic Surg Lasers Imaging Retina. 2020;51:11:633-639.
5. Almony A, Keyloun KR, Shah-Manek B, et al. Clinical and economic burden of neovascular age-related macular degeneration by disease status: A US claims-based analysis. J Manag Care Spec Pharm. 2021;27:9:10.
6. Keenan TDL, Goldstein M, Goldenberg D, Zur D, Shulman S, Loewenstein A. Prospective, longitudinal pilot study. Ophthalmology Science. 2021;1:2:100034.
7. Blinder KJ, Calhoun C, Maguire MG, et al. Home OCT imaging for newly diagnosed neovascular age-related macular degeneration: A feasibility study. Ophthalmology Retina. 2024;8:4:376-387.
8. Holekamp NM, de Beus AM, Clark WL, Heier JS. Prospective trial of home optical coherence tomography–guided management of treatment experienced neovascular age-related macular degeneration patients. Retina. 2024;44:10:1714.



