 |
|
Bios Mr. Sriranganathan is a medical student at the University of Ottawa, Ottawa, Ontario, Canada. Dr. Mandelcorn is vitreoretinal surgeon and the chief of ophthalmology at Toronto Western Hospital. Dr. Felfeli is an ophthalmology resident at the University of Toronto, Toronto, Ontario, Canada. |
Uveitis presents as inflammation of the eye’s uveal tissue and belongs to a spectrum of vision-threatening diseases accounting for 2.8 to 10 percent of all cases of blindness worldwide. Two-thirds of patients with non-infectious uveitis experience prolonged vision loss.1 Complications of NIU include glaucoma, corneal deposition, cataract and macular edema.2,3
NIU burden impacts the daily functioning of individuals in a variety of ways, such as job insecurity and increased stress levels.2 Affecting all age groups, the burden of NIU touches multiple facets of patients’ lives from an individual, health-care system and societal perspective. Given the increase in recent literature evaluating the disease burden of NIU, we deemed that a synthesis of the global impact of NIU was warranted.
Here, we’ll share some trends and findings in recent NIU literature.
Incidence and prevalence
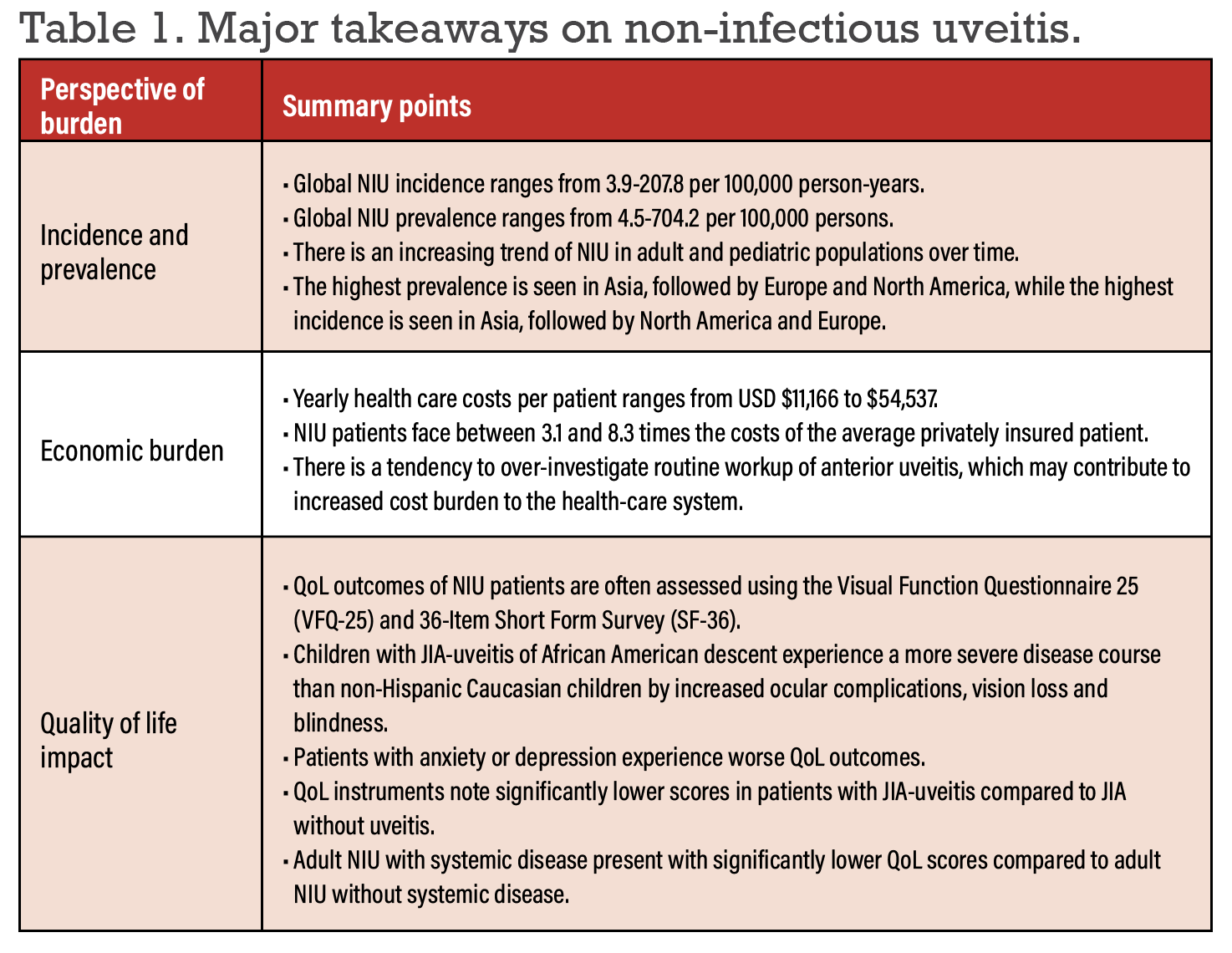
Recent epidemiological studies have revealed that NIU incidence ranges from 3.9 to 207.8 per 100,000 person-years and the prevalence ranges from 4.5 to 704.2 per 100,000 persons.4-7 Pediatric NIU ranges from 4.6 to 7.3 per 100,000 person-years and the prevalence ranges from 8.3 to 106 per 100,000 persons.
Recent studies have shown an increasing prevalence of NIU over time. A study by Kazuhiko Umazume, MD, and co-authors reported a change from 386.5 in 2012 to 439.3 per 100,000 persons in 2016.5 Likewise, Oulu, Finland’s Mira Siiskonen, MD, and colleagues reported a change from 64 in 2008 to 106 per 100,000 persons in the pediatric population.8
A retrospective study that evaluated the association between air pollution and NIU in Taiwan reported an incidence of 1,256.49 cases in a population of 100,000 followed over 11 years. Air pollution was significantly associated with incidental uveitis, particularly at higher total hydrocarbon, methane and nitrogen oxide levels.9
An evaluation of NIU attacks compared by seasons noted a higher incidence of NIU attacks in the winter than autumn, with significant association to the number of rainy days and average wind speed per month.10
Recent literature has shown variable epidemiological outcomes of NIU across different contexts, including adults vs. pediatrics, comorbid systemic disease, hospitalization, various subtypes of NIU, various intervention outcomes and during the COVID-19 pandemic. Among systemic diseases, the most common associations were of juvenile idiopathic arthritis, ankylosing spondylitis, spondyloarthritis, psoriatic arthritis and
Behçet disease.
Studies have demonstrated the highest NIU prevalence in Asia, followed by Europe and North America, while the highest incidence was in Asia, followed by North America and Europe. Asian countries often see higher rates of BD and Vogt-Koyanagi-Harada Disease, while Western countries see higher rates of AS, PsA and SpA.
 |
Economic burden
The economic burden of NIU relies on direct medical costs, intervention and medication costs, direct medical resource use, indirect resource use and costs, and adverse event costs. Most cost-burden studies detail direct medical costs, such as procedural, inpatient, outpatient, visitation, emergency room and investigation costs. Fewer studies reported intervention and medication costs (e.g., prescription drug and pharmacy service costs) and medical resource use (e.g., number of inpatient and outpatient visits, time for service acquisition).
Average annual health-care costs ranged from $11,166 to $54,537.11 In 2009, the average cost of NIU management ranged from 3.1 to 8.3 times the cost of the average privately insured patient in the United States.11
A study of health-care utilization for NIU patients undergoing different therapies noted that monthly per-patient-per-month costs of corticosteroids, immunosuppressants and biologics were $935, $1,738 and $1,439, respectively.12 While immunosuppressants and biologics were associated with improvements in ophthalmic symptoms, hospital admission rates and ER visits, corticosteroids were associated with increases in these measures, suggesting that corticosteroids may be an overused therapy for NIU.12
Economic burden is heavily associated with quality of life outcomes. Economic hardship was found to be a significant factor that contributed to poor mental health outcomes in patients with NIU.13 Work discontinuation due to NIU burden contributes to the economic burden of individuals, with a disproportionate impact in individuals of low- and middle-income countries. Service provisions alleviate some of the burden on individual from more affluent countries.14
Cost-effectiveness evidence
A growing trend in economic evaluations is cost-effectiveness and cost-utility studies. NIU is often managed with systemic corticosteroids and immunosuppressants, such as adalimumab, methotrexate and mycophenolate mofetil. The cost-effectiveness of triamcinolone acetonide for suprachoroidal injection, dexamethasone implants and fluocinolone acetonide implants have also been assessed.
Recent studies have suggested that adalimumab may be a more cost-effective option than current practice guidelines for patients with active uveitis at greater risk of blindness.15–18 A study by researchers at the University of Sheffield19 suggested that dexamethasone is a cost-effective option compared to limited current practice for uveitis management. Likewise, the MUST Trial20 concluded that fluocinolone acetonide implant therapy was a cost-effective option compared to systemic therapy in non-contraindicated and treatment failure contexts.
A recent study of clinical practice patterns in the context of NIU revealed that there’s a tendency to over-investigate routine workup of anterior uveitis in Canada.21 Adhering to clinical practice guidelines may result in cost savings of $600,000 per year to the Canadian health-care system.
Humanistic burden
The QoL of patients with NIU have been evaluated through a number of QoL instruments, including the Visual Function Questionnaire 25, 36-Item Short Form Survey, EuroQol 5D and Pediatric Quality of Life. QoL outcomes were often compared across age groups, comorbid systemic diseases and various treatment modalities. Fourteen QoL instruments were employed across studies evaluating the humanistic burden of NIU, with the majority using VFQ-25 followed by SF-36.
Recent studies have investigated the QoL outcomes of patients with NIU in pediatric populations, in populations with systemic disease and in populations undergoing systemic medical therapies. Studies assessing QoL are most often conducted in Europe, followed by North America and Asia.
In a study comparing racial differences of QoL outcomes in patients with JIA-associated uveitis, researchers22 noted that children with JIA-uveitis of African-American descent experience a more severe disease course than non-Hispanic Caucasian children by increased ocular complications, vision loss and blindness.
A qualitative thematic analysis in Australia23 noted that aside from recognized challenges and difficulties faced by populations with vision impairments, NIU patients face additional challenges such as prognostic uncertainty and associated discomfort, and concern regarding inflammatory relapses.
Another thematic study on pediatric NIU populations reported that themes including “impact on school,” “social factors” and “emotional reactions” are significant predictors of poor QoL outcomes.24
QoL outcomes were further significantly reduced in NIU patients with anxiety or depression compared to NIU patients without.25
Studies comparing QoL outcomes of JIA-uveitis and non-uveitic JIA note significantly lower QoL in patients with JIA-uveitis. Other studies assessing QoL outcomes of adult NIU with systemic disease vs. healthy controls report significantly lower scores among adult NIU participants with systemic disease
Bottom line
Given the significant burden of NIU from an economic, humanistic and epidemiological standpoint, it’s important for ophthalmologists to have an understanding of the unique experience of this complex disease, including its subtypes and systemic comorbidities. It’s also important to continue to find cost-effective therapy options, address QoL predictors and alleviate barriers of NIU patients in their pursuit to normalcy. Finally, the scarcity of evidence evaluating the burden of NIU in non-Westernized countries warrants further studies in these geographical regions. RS
REFERENCES
1. Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:9:1159-1162.
2. Hoeksema L, Los LI. Vision-related quality of life in herpetic anterior uveitis patients. PLOS ONE. 2014;9:1:e85224.
3. Herbort CP, Rao NA, Mochizuki M, members of Scientific Committee of First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: Results of the first International Workshop on Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm. 2009;17:3:160-169.
4. Bro T, Tallstedt L. Epidemiology of uveitis in a region of southern Sweden. Acta Ophthalmol (Copenh). 2020;98:1:32-35.
5. Umazume A, Ohguro N, Okada AA, et al. Prevalence and incidence rates and treatment patterns of non-infectious uveitis in Japan: Real-world data using a claims database. Jpn J Ophthalmol. 2021;65:5:657-665.
6. Reid G, Williams M, Compton M, Silvestri G, McAvoy C. Ocular sarcoidosis prevalence and clinical features in the Northern Ireland population. Eye. 2022;36:10:1918-1923.
7. Hermann L, Falcão-Reis F, Figueira L. Epidemiology of uveitis in a tertiary care centre in Portugal. Semin Ophthalmol. 2021;36:1-2:51-57.
8. Siiskonen M, Hirn I, Pesälä R, Hautala T, Ohtonen P, Hautala N. Prevalence, incidence and epidemiology of childhood uveitis. Acta Ophthalmol (Copenh). 2021;99:2:e160-e163.
9. Bai YC, Wang CY, Lin CL, Lai JN, Wei JCC. Association between air pollution and the risk of uveitis: A nationwide, population-based cohort study. Front Immunol. 2021;12:613893.
10. Gómez-Mariscal M, De Arriba F, Revenga M, González-López JJ. Do season and environment have a role in the incidence of anterior uveitis attacks? Ocul Immunol Inflamm. 2020;28:5:786-790.
11. Albini TA, Rice JB, White AG, et al. Economic burden of non-infectious inflammatory eye disease (NIIED) in a commercially-insured population in the United States. Ocul Immunol Inflamm. 2020;28:1:164-174.
12. Chu DS, Johnson SJ, Mallya UG, Davis MR, Sorg RA, Duh MS. Healthcare costs and utilization for privately insured patients treated for non-infectious uveitis in the USA. J Ophthalmic Inflamm Infect. 2013;3:1:1-10.
13. Jin Y, Lin D, Dai ML, et al. Economic hardship, ocular complications, and poor self-reported visual function are predictors of mental problems in patients with uveitis. Ocul Immunol Inflamm. 2021;29:6:1045-1055.
14. Uutela A. Economic crisis and mental health. Curr Opin Psychiatry. 2010;23:2:127.
15. Bermejo I, Squires H, Poku EN, et al. Adalimumab for non-infectious uveitis: Is it cost-effective? Br J Ophthalmol. 2019;103:11:1633-1638.
16. Hughes DA, Culeddu G, Plumpton CO, et al. Cost-effectiveness analysis of adalimumab for the treatment of uveitis associated with juvenile idiopathic arthritis. Ophthalmology. 2019;126:3:415-424.
17. Martín-Varillas JL, Calvo-Río V, Beltrán E, et al. Successful optimization of adalimumab therapy in refractory uveitis due to Behçet’s disease. Ophthalmology. 2018;125:9:1444-1451.
18. Ramanan AV, Dick AD, Jones AP, et al. Adalimumab in combination with methotrexate for refractory uveitis associated with juvenile idiopathic arthritis: A RCT. Health Technol Assess Winch Engl. 2019;23:15:1-140.
19. Squires H, Bermejo I, Poku EN, et al. Dexamethasone implant for non-infectious uveitis: Is it cost-effective? Br J Ophthalmol. 2019;10311:1639-1644.
20. Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, et al. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: Fifty-four-month results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and follow-up study. Ophthalmology. 2015;122:10:1967-1975.
21. Noble J, Hollands H, Forooghian F, et al. Evaluating the cost-effectiveness of anterior uveitis investigation by Canadian ophthalmologists. Can J Ophthalmol J Can Ophtalmol. 2008;43:6:652-657.
22. Angeles-Han ST, McCracken C, Yeh S, et al. Characteristics of a cohort of children with juvenile idiopathic arthritis and JIA-associated uveitis. Pediatr Rheumatol Online J. 2015;13:101248897:19.
23. Prem Senthil M, Lim L, Braithwaite T, et al. The impact of adult uveitis on quality of life: An exploratory study. Ophthalmic Epidemiol. 2021;28:5:444-452.
24. Sen ES, Morgan MJ, MacLeod R, et al. Cross sectional, qualitative thematic analysis of patient perspectives of disease impact in juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J. 2017;15:58.
25. Vakros G, Scollo P, Hodson J, Murray PI, Rauz S. Anxiety and depression in inflammatory eye disease: Exploring the potential impact of topical treatment frequency as a putative psychometric item. BMJ Open Ophthalmol. 2021;6:1:e000649.




