Take-home points
|
 |
Bios Dr. Shughoury is a uveitis fellow at the Northwestern University Feinberg School of Medicine in Chicago. Dr. Ciulla is a retina specialist and board member of Midwest Eye Institute of Indianapolis, Indiana; volunteer clinical professor of ophthalmology at Indiana University School of Medicine; chief medical officer at Viridian Therapeutics; and chief medical advisor and the chair of the Scientific Advisory Board at Clearside BioMedical, where he previously served as chief medical officer and chief development officer. DISCLOSURES: Dr. Shughoury reports no relevant disclosures. Dr. Ciulla reports employment by, and holds equity in, Viridian Therapeutics. He holds equity in Clearside BioMedical and Nanoscope Therapeutics. In addition to Clearside and Nanoscope, he has served as a consultant for Ocuphire Pharma. |
A Real-World Look at GA
Geographic atrophy secondary to age-related macular degeneration presents significant challenges for both patients and retinal specialists. Patients grapple with the steady loss of central vision and progressive visual impairment, while clinicians have historically lacked therapeutic options beyond routine monitoring. New intravitreal complement-inhibitors such as pegcetacoplan and avacincaptad pegol may slow GA progression, but it remains unclear which patients are ideal candidates for long-term, monthly therapy. Additionally, these novel treatments are known to increase the risk of vision-threatening exudation in eyes with GA,1 necessitating careful assessment of risks and benefits before starting treatment.
Data from the placebo arms of randomized trials have shed some light on the natural course of untreated GA, but real-world patient populations often differ meaningfully from those enrolled in trials. Our recent study, Three-Year Clinical Outcomes in Geographic Atrophy: An Analysis of 18,712 Eyes,2 seeks to clarify the natural history of GA in daily retinal practice to better inform our approach to patient care. By analyzing clinical data gathered from hundreds of U.S. retinal specialists across the country over several years of follow-up, our study aims to provide some of the data necessary to have more nuanced, patient-centered discussions around GA prognosis and the decision to initiate therapy.
Data collection and analysis
To evaluate real-world outcomes in GA, we utilized the CorEvitas Vestrum Health Retina Database, a large retina-specific database that compiles de-identified patient records from more than 350 retina specialists located in diverse practice settings and geographic regions across the United States. We specifically focused on the eyes of new patients presenting to retinal specialty care who received a diagnosis of GA between October 2015 and August 2022 and had 36 months of follow-up data on file. Eyes with a preexisting diagnosis of neovascular AMD were excluded from the study. Collected data ranged from basic demographics to visual acuity, central subfield thickness on optical coherence tomography and the presence or absence of AMD pathology in the fellow eye. Analysis was performed at the patient eye level unless otherwise specified. Visual acuity analysis was performed using ETDRS letter scores, and risk of conversion to nAMD was determined on the basis of ICD-10 coding changes. By focusing on these parameters, we aimed to provide insights into the functional impact of GA progression and the potential for exudative complications.
Baseline patient characteristics
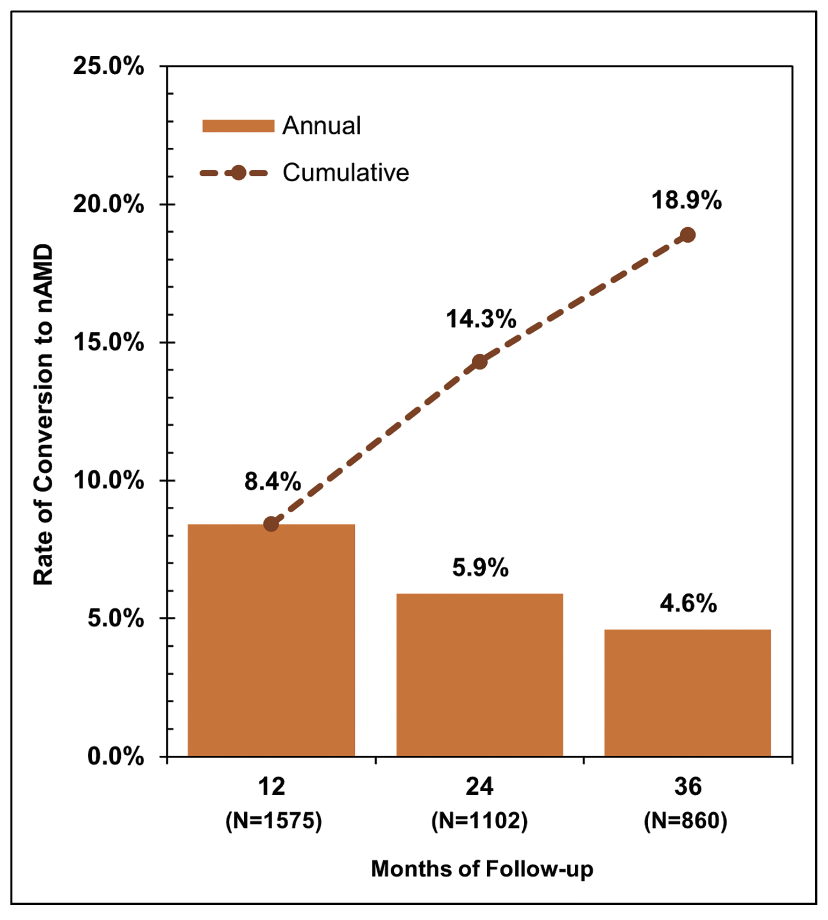 |
| Figure 1. Overall rate of conversion to neovascular age-related macular degeneration among eyes with geographic atrophy across three years of follow up. |
A total of 18,712 eyes met criteria for inclusion in the study. Mean age at presentation was approximately 78.6 years, with 66 percent of eyes belonging to female patients. Nearly all eyes with GA (98.8 percent) had some form of AMD in the fellow eye. Approximately 44 percent of eyes had relatively good VA at baseline (VA ≥ 20/40), 33 percent had moderate baseline visual impairment (VA < 20/40 and ≥ 20/100), and 8 percent had severe baseline visual impairment (VA < 20/100 and ≥ 20/200). Another 14 percent had baseline VA < 20/200 (legally blind) and were excluded from visual outcomes analyses due to difficulty precisely quantifying very low vision in letter scores.
Who’s at risk of developing neovascular AMD?
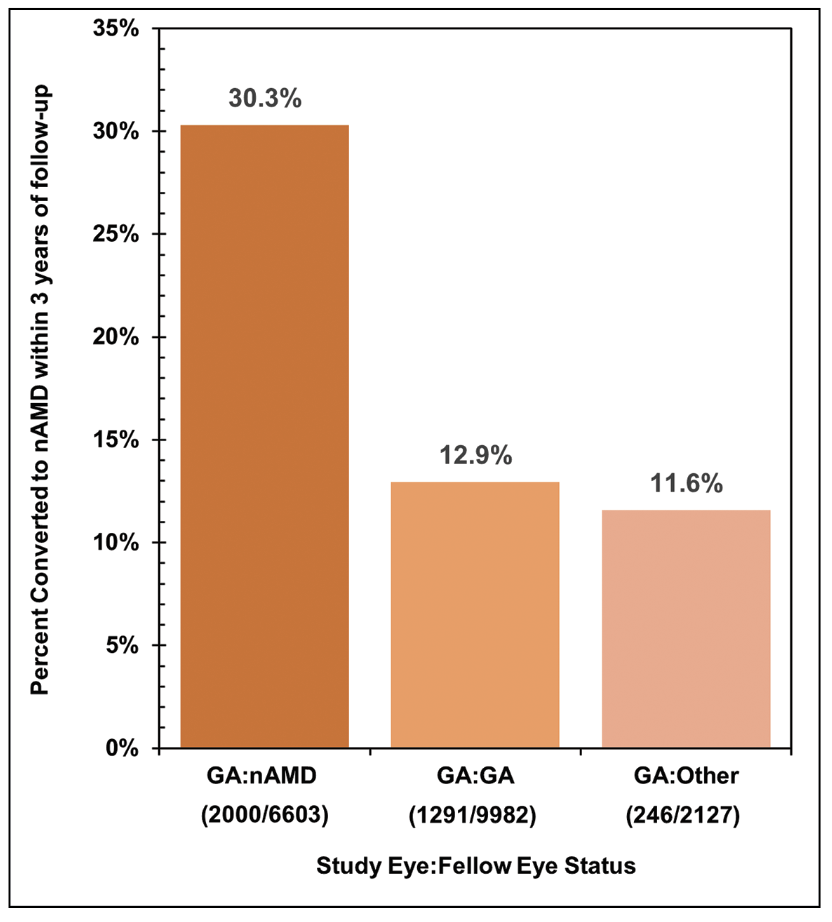 |
| Figure 2. Rate of developing neovascular age-related macular degeneration within three years of follow-up among eyes with contralateral geographic atrophy (GA:GA), nAMD (GA:nAMD), or neither GA nor nAMD (GA:Other). |
Approximately one in five eyes (18.9 percent) developed nAMD within the first three years of presenting to retinal specialty care with GA (Figure 1). This rate aligns with the observations of previous real-world studies,3,4 but is markedly higher than rates reported in placebo arms of the p egcetacoplan and avacincaptad pegol trials.1,5–7 The discrepancy is likely due to the fact that clinical trials typically employ rigorous eligibility criteria and comprehensive imaging to exclude any evidence of exudation or subclinical choroidal neovascularization at baseline. Because such detailed screening isn’t commonly performed in routine clinical practice, such eyes may not be identified as having nAMD at the initial retina visit. Nevertheless, our findings may more realistically represent the incidence of nAMD detection among eyes with GA in everyday retina practice as compared to clinical trials, highlighting the need for careful monitoring and close follow-up.
We also found that the presence of nAMD in the fellow eye was a strong predictor of new-onset exudation in the eye with GA (Figure 2). Eyes with fellow-eye nAMD converted at more than twice the rate of those with fellow-eye GA (p<0.001) or those with neither GA nor nAMD in the fellow eye (p<0.001). Previous real-world studies have similarly suggested that GA eyes with fellow-eye nAMD are at heightened risk of developing nAMD.3 Patients with GA who have evidence of nAMD in the contralateral eye therefore ought to receive vigilant surveillance to allow early detection of vision-threatening exudation and prompt intervention.
How rapidly does vision decline in GA?
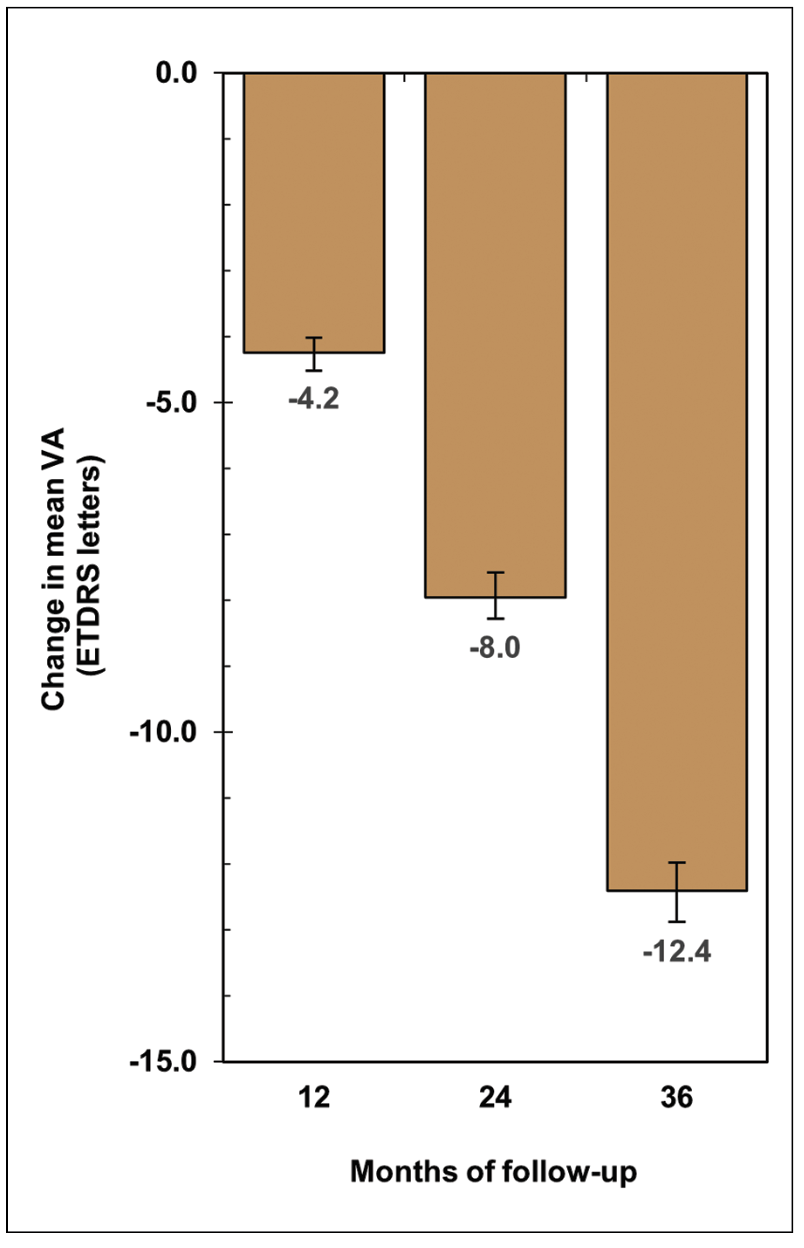 |
| Figure 3. Overall change in mean visual acuity over three years in eyes with geographic atrophy. |
Among GA eyes that didn’t develop nAMD, we observed a mean loss of 12.4 letters of VA—two to three lines—over three years, at a pace of about four letters per year (Figure 3). This rate is in agreement with that reported in previous real-world studies.3,4,8 By the three -year mark, only half of the eyes that presented with driving-level vision (VA ≥ 20/40) maintained it, while approximately 18 percent had progressed to low vision (VA ≤ 20/70), and 8 percent could be categorized as legally blind (VA ≤ 20/200). Patients with GA are therefore at high risk of developing debilitating vision loss within only a few years of follow up.
Who’s most vulnerable to vision loss?
In our study, older age at presentation was associated with accelerated vision loss and worse visual outcomes at each year of follow-up (Figure 4). Eyes of patients in the oldest age quartile (≥86 years) declined at roughly double the rate of those in the youngest quartile (≤74 years). By three years, fewer than 20 percent of eyes in the oldest age quartile had driving-level vision, while 32 percent were legally blind.
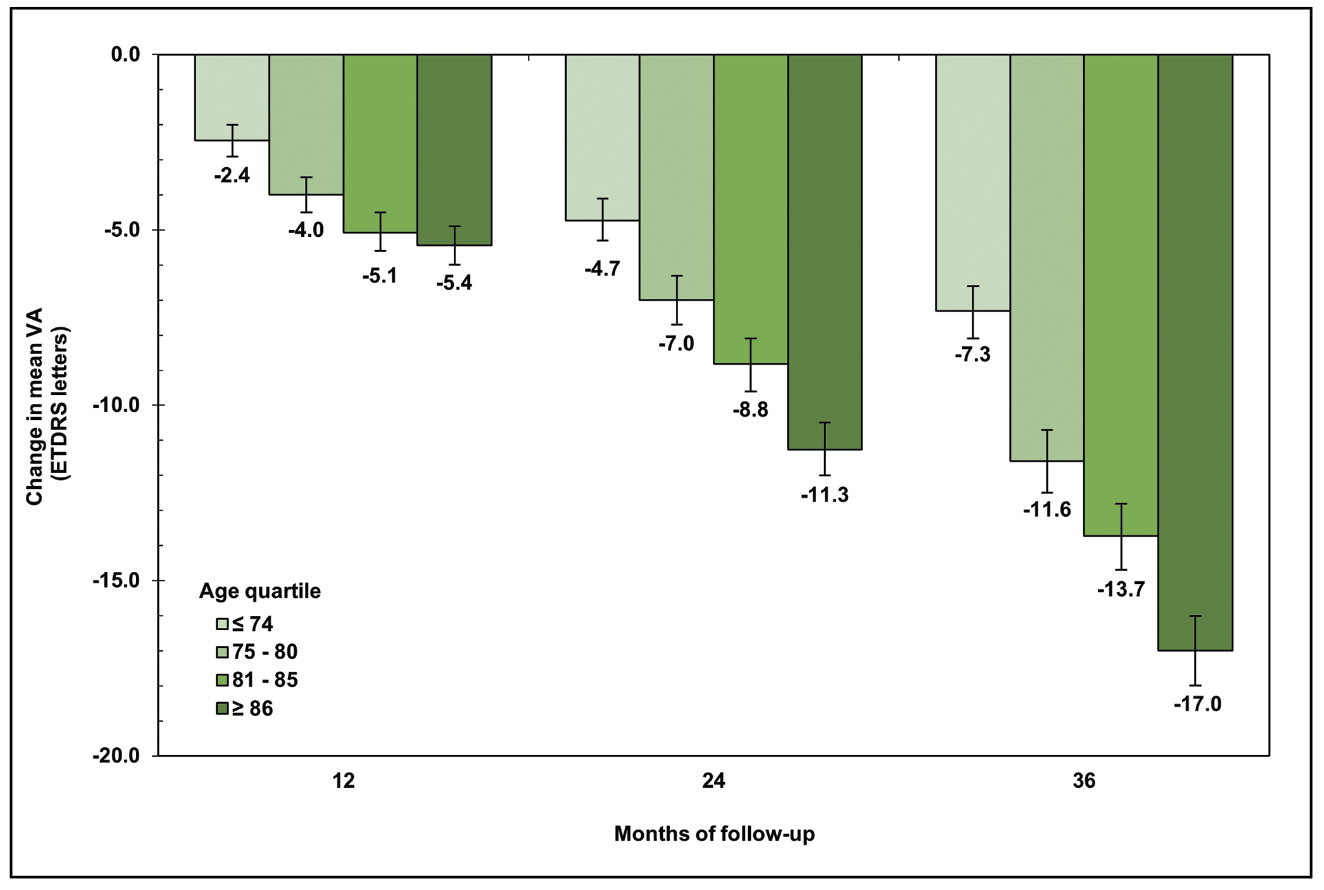 |
| Figure 4. Change in mean visual acuity over three years in eyes with geographic atrophy, stratified by age quartile. |
We also observed a non-linear relationship between baseline severity of visual impairment and degree of vision loss (Figure 5). Interestingly, eyes with moderate baseline visual impairment (VA < 20/40 to 20/100) experienced the most pronounced decline in mean VA, losing around three lines (15.6 letters) by the three-year mark. In contrast, eyes with minimal baseline visual impairment (VA ≥ 20/40) or severe baseline visual impairment (VA < 20/100 to 20/200) declined together at substantially lower rates, losing only two lines over three years. This phenomenon may represent progression from juxtafoveal to subfoveal GA in eyes with moderate baseline impairment, as opposed to a ceiling effect among eyes with early disease and a floor effect among those with more advanced GA.
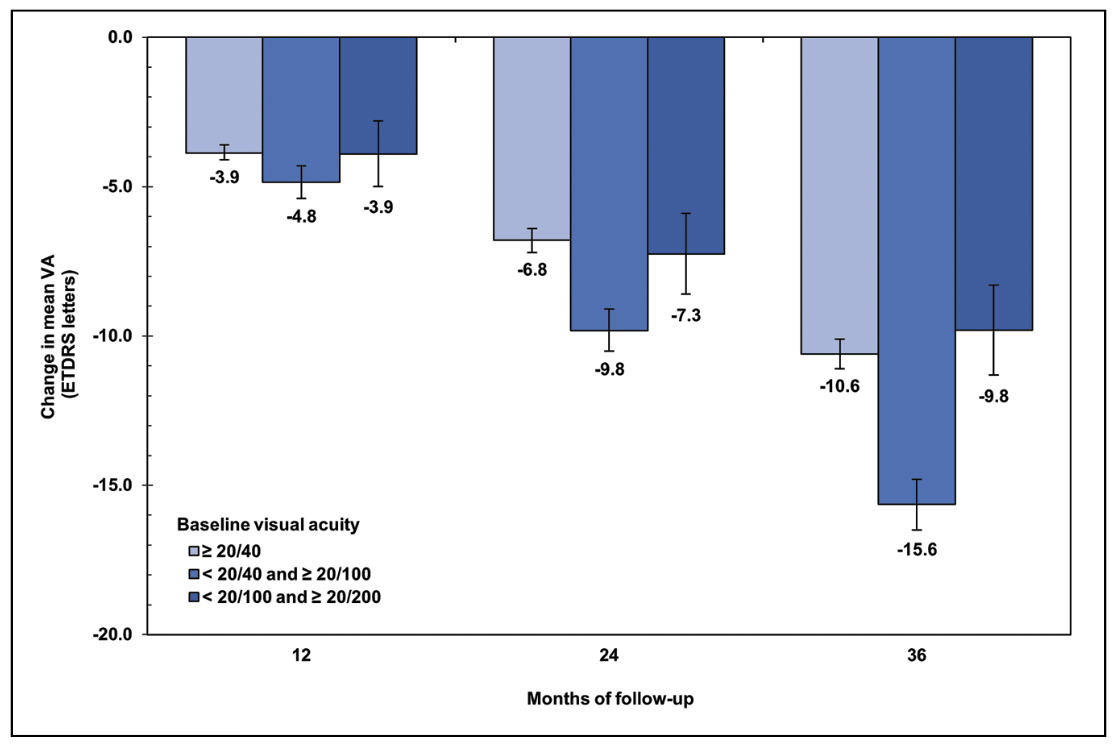 |
| Figure 5. Change in mean visual acuity over three years in eyes with geographic atrophy, stratified by baseline degree of visual impairment. |
Additionally, multivariable analysis revealed that baseline age and baseline visual acuity correlate independently with degree of vision loss in GA. On combined stratified analysis, the greatest degree of vision loss was identified among eyes of patients ≥86 years old with moderate baseline visual impairment (Figure 6). These eyes lost approximately 20 letters (four lines) on average over three years, at a rate of just over one line (6.4 letters) per year. Conversely, eyes of young patients ≤74 years old with minimal visual impairment lost only around one line of VA on average over the three years of follow up, while eyes of young patients ≤74 years with severe baseline visual impairment didn’t experience significant decline.
CST tells only part of the story
We also assessed available CSTs on OCT among GA eyes that didn’t convert to nAMD. Despite losing an average of more than two lines of VA as above, these eyes didn’t demonstrate clinically significant CST changes at three years. We know that GA typically spares the foveal center until advanced stages of disease, so it’s no surprise that standard thickness measurements don’t always align with functional loss. Nevertheless, OCT imaging remains an important tool to monitor GA for anatomic progression and new-onset exudation.
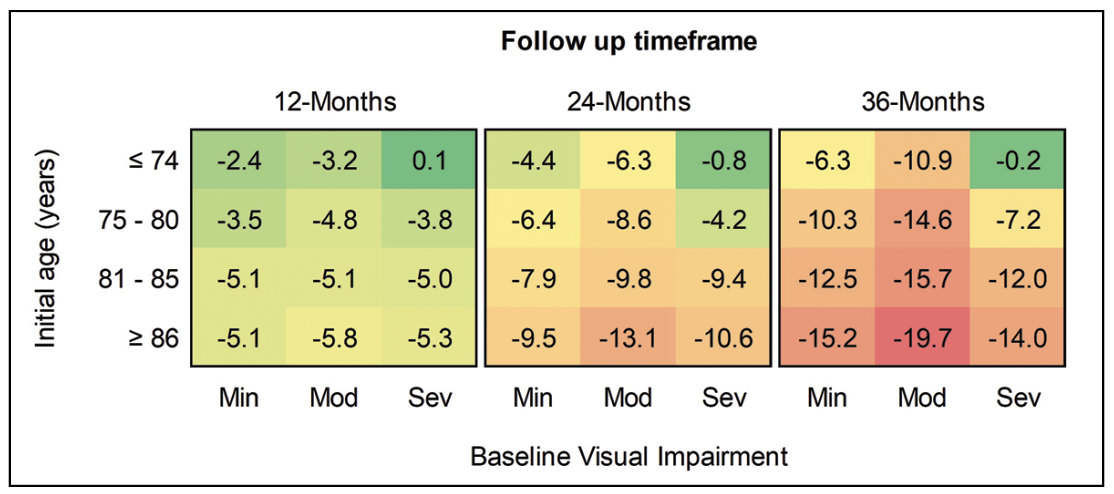 |
| Figure 6. Heatmap of change in mean visual acuity over three years in eyes with geographic atrophy, stratified by age quartile and baseline degree of visual impairment. Min = Baseline VA ≥ 20/40; Mod = Baseline VA < 20/40 and ≥ 20/100; Sev = Baseline VA < 20/100 and ≥ 20/200. |
Strengths and limitations
By analyzing a diverse range of thousands of patients under retinal specialty care over three years of follow-up, our study aims to provide insights into the natural history of GA in real-world retina clinics. However, like any retrospective registry-based study, ours has important limitations. For example, data collection methods weren’t standardized, and variability in diagnostic coding practices and screening protocols may contribute to potential misclassification or underdiagnosis of subtle neovascular disease that skew the results. Nevertheless, limiting our analysis to patients under retina specialty care may have mitigated some of these issues, as patients are likely to receive more detailed evaluations and accurate diagnoses when managed by clinicians with subspecialty expertise.
How should we counsel our patients?
Our clinical approach to GA is changing rapidly. Novel intravitreal therapeutics to slow GA progression have begun altering the conversations we have with our patients, shifting focus from observation to active treatment. The findings of our study suggest that older patients and those presenting with moderate visual impairment may be at highest risk of rapid vision loss, and may stand to benefit most from therapy to slow disease progression. However, the presence of nAMD in the contralateral eye should warrant a very careful discussion of risks and benefits before initiating therapy that may increase risk of exudation.
In addition to therapeutic decision-making, clinicians should consider proactively counseling patients with GA to help them understand and navigate their prognosis. Key strategies include:
Patient education. Explaining the anticipated trajectory of GA—especially the possibility of exudation—can help patients set realistic long-term expectations and understand the importance of close follow-up.
Low-v ision rehabilitation. Given that a significant proportion of eyes with GA will decline to a VA of ≤ 20/70 within a few years of follow-up, earlier engagement with low-vision services can provide patients with tools to manage daily tasks, preserve independence and maintain quality of life as GA progresses.
Consistent OCT imaging. With a near-20-percent chance of developing nAMD within three years of presenting to retina specialty care, home monitoring (e.g. Amsler grid) and routine OCT surveillance are critical for catching neovascular changes early, especially in patients who have nAMD in the contralateral eye.
Bottom line
This three-year analysis of 18,712 eyes with untreated GA in real-world practice offers key insights into the natural history of disease that can help guide the clinical conversations we have with our patients. These patients face a significant risk of rapid and meaningful vision loss within the first few years of presenting to retinal specialty care.
Patients with GA lose about one line of acuity per year on average, although older age and moderate visual impairment are independently associated with faster decline and worse visual outcomes. Additionally, eyes with GA are at high risk of developing nAMD, particularly in the presence of contralateral neovascular disease, underscoring the need for close follow-up and vigilant monitoring.
As new treatments for GA expand our therapeutic toolkit, these insights enable us to have more personalized, evidence-based discussions with our patients about prognosis, treatment options and potential risks. Moreover, integrating these considerations into everyday clinical practice can help us better support our patients with GA as we navigate their challenging diagnosis together. RS
REFERENCES
1. Wykoff CC, Rosenfeld PJ, Waheed NK, et al. Characterizing new-onset exudation in the randomized Phase 2 FILLY Trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology. 2021;128:9:1325-1336.
2. Shughoury A, Boucher N, Aggarwal N, Ciulla TA. Three-year clinical outcomes in geographic atrophy: An analysis of 18,712 patient eyes. RETINA. 2025;45:2:188-197.
3. Rahimy E, Khan MA, Ho AC, et al. Progression of geographic atrophy: Retrospective analysis of patients from the IRIS Registry (Intelligent Research in Sight). Ophthalmology Science. 2023;3:4:100318.
4. Chakravarthy U, Bailey CC, Johnston RL, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:6:842-849.
5. Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: A randomized Phase 2 trial. Ophthalmology. 2020;127:2:186-195.
6. Patel SS, Lally DR, Hsu J, et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye. 2023;37:17:3551-3557
7. Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, Phase 3 trial. The Lancet. 2023;402:10411:1449-1458.
8. Heier JS, Pieramici D, Chakravarthy U, et al. Visual function decline resulting from geographic atrophy: Results from the Chroma and Spectri Phase 3 trials. Ophthalmology Retina. 2020;4:7:673-688.



