Take-home points
|
 |
Bios Dr. Rusakevich is a second-year ophthalmology resident at University of California Davis Health in Sacramento. Dr. Yiu is a professor of ophthalmology at UC Davis, where he is a vitreoretinal surgeon and clinician-scientist. Disclosures: Dr. Rusakevich has no relevant relationships to disclose. Dr. Yiu disclosed relationships with 4D Molecular Therapeutics, AbbVie, Adverum, Alimera Sciences, Bausch + Lomb, Boehringer Ingelheim, Clearside Biomedical, Endogena, Genentech/Roche, Gyroscope Therapeutics, Iridex, Janssen, jCyte, Myrobalan, NGM Biopharmaceuticals, Novartis, Ray Therapeutics, |
Updated statistics show that age-related macular degeneration is expected to affect 288 million people worldwide by the year 2040.1 Anti-vascular endothelial growth factor therapy has revolutionized and remains the cornerstone of neovascular AMD management, but frequent dosing is costly and time-consuming for both patients and providers.2 Ongoing efforts to reduce the treatment burden have included treat-and-extend dosing regimens, more durable pharmacologic agents, alternative drug delivery systems and gene therapy.
Meanwhile, inherited retinal diseases caused by single-gene mutations, such as RPE65 in Leber congenital amaurosis and retinitis pigmentosa, have shown promising results with gene therapy treatment. This led to the Food and Drug Administration in 2017 to approve voretigene neparvovec (Luxturna, Spark Therapeutics) for biallelic RPE65 mutation-associated retinal diseases.3
Complex retinal conditions such as AMD pose a more challenging target for gene therapy because of the complex interplay of many gene associations and environmental factors contributing to its pathogenesis. In recent years, however, gene therapy is emerging as a promising strategy to serve as a biofactory approach for long-term production of VEGF or complement inhibitors.
Early gene therapy for exudative AMD
Early exploration of gene therapy for nAMD was based on the premise of subretinal delivery of an adeno-associated virus (AAV) vector to enable long-term expression of a VEGF inhibitor using host cell machinery. Ian Constable, MBBS, and colleagues4 first proposed subretinal delivery of an AAV to express soluble VEGF receptor (sFLT-1) for nAMD. Their Phase IIa investigation included 21 participants in the gene therapy treatment arm and showed no serious side effects to meet the primary endpoint for safety.
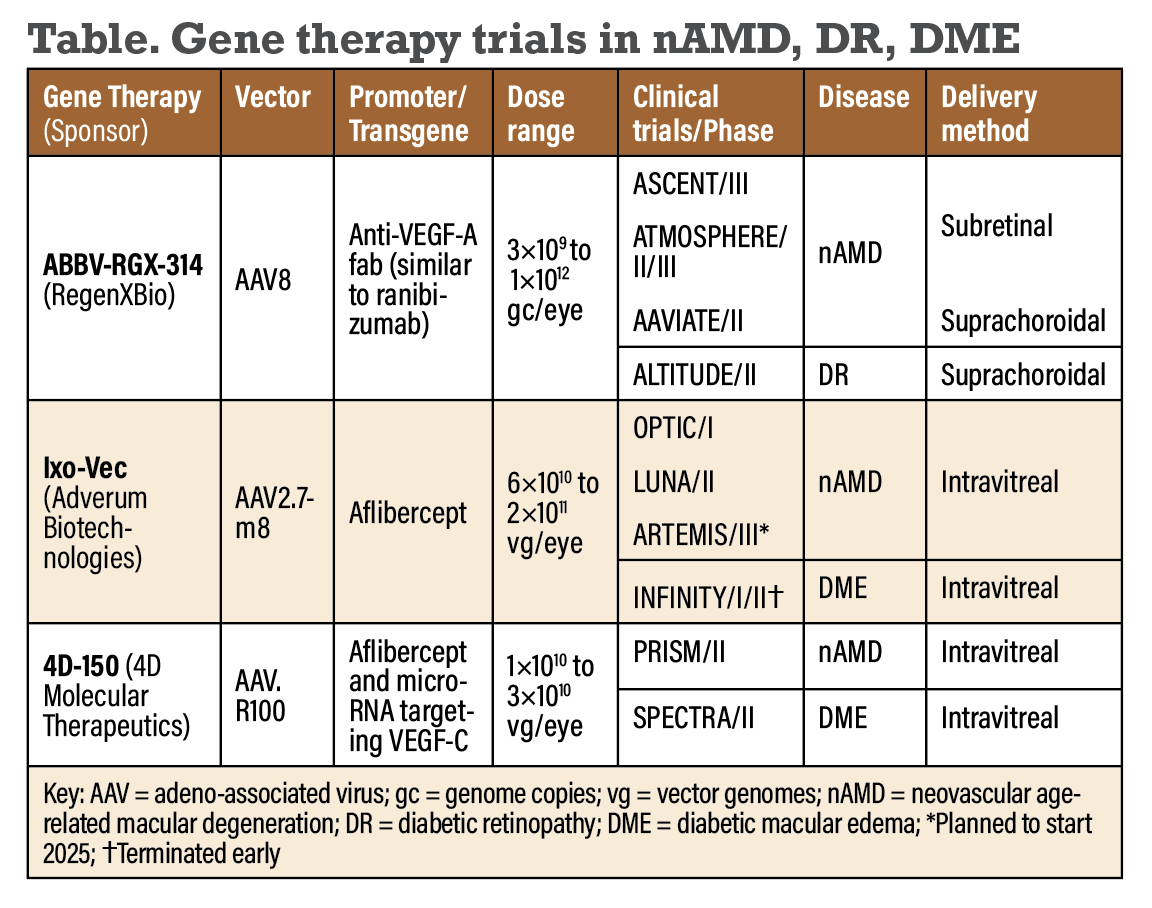
Neither visual acuity nor the number of anti-VEGF rescue treatments were statistically different between the treatment and control groups, however, which curtailed its further development.4 Despite these disappointing early results, several exciting new gene therapy programs under development are showing greater promise (Table below).
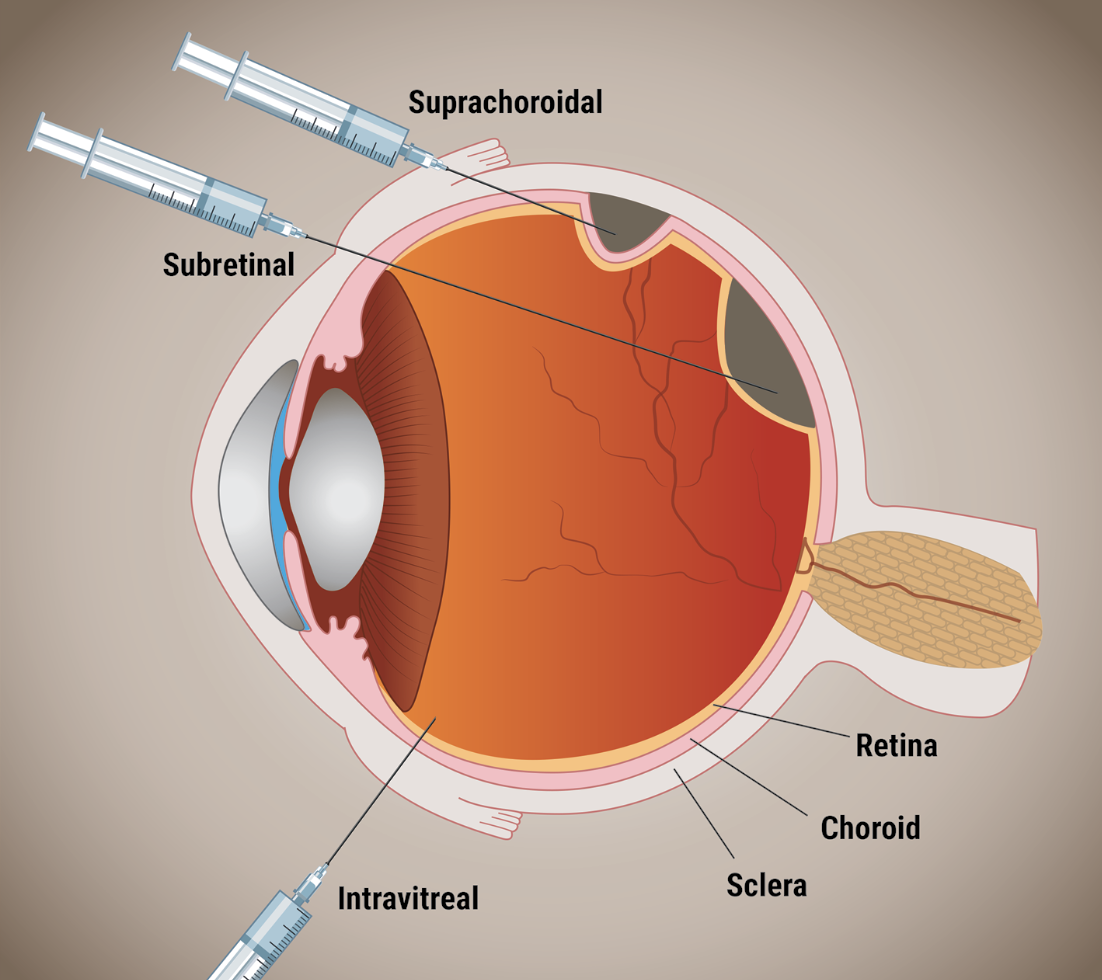 |
| The three routes of administering gene therapy for neovascular age-related macular degeneration. |
Subretinal ABBV-RGX-314
The most advanced development program to date is ABBV-RGX-314 (RegenXBio), an AAV serotype 8 vector that’s administered into the subretinal space and expresses an anti-VEGF-A antigen-binding fragment similar to ranibizumab. The procedure involves a standard three-port, small-gauge pars plana vitrectomy, induction of a posterior vitreous detachment and subretinal injection of 0.2 mL of the test product using a 41-gauge subretinal cannula, followed by a partial air-fluid exchange.
The open-label, multicenter Phase I/IIa trial showed sustained gains in best-corrected visual acuity, few rescue injections and durable aqueous protein concentrations among 42 patients over long-term follow up of more than three years. Primary safety results demonstrated dose-dependent retinal pigmentary changes, but no significant treatment-associated ocular inflammation.5 The program is now recruiting for two pivotal randomized clinical trials, ASCENT and ATMOSPHERE, to evaluate two dose levels of ABBV-RGX-314 vs. intravitreal ranibizumab and aflibercept injection schedules.6,7
Intravitreal Ixo-Vec
Because naturally occurring AAV vectors can’t transduce the retina when administered intravitreally, newer generations of engineered vectors have been developed to avoid the need for vitreoretinal surgery. Deniz Dalkara, PhD, and colleagues at the University of California, Berkeley, described a process known as “directed evolution,”8 which involves injecting diverse AAV capsid libraries intravitreally into mouse eyes. Successful variants are extracted from harvested photoreceptors, sequenced and amplified, and then used to create new libraries for additional rounds of selection to develop novel capsid variants, such as AAV2.7m8, capable of overcoming the internal limiting membrane barrier and transduce the entire retina from the vitreous cavity.
Ixoberogene soroparvovec (ixo-vec, formerly ADVM-022, Adverum Biotechnologies) is a gene therapy that uses the AAV2.7m8 capsid to express the aflibercept protein.9 In the OPTIC first-in-human study, 30 patients with nAMD requiring frequent anti-VEGF injections (about 10 annually)received one of two ixo-vec doses—2×1011 or 6×1011 vector genomes (vg)/eye—and prophylactic steroids (either topical difluprednate or oral prednisone).
In these previously treated patients with high injection burden, a single ixo-vec treatment provided 80-to-98-percent reduction in annualized anti-VEGF treatments.10 A recent four-year analysis of OPTIC data demonstrated that half of patients remained anti-VEGF injection-free through the end of year four.11
Interim 52-week data from the ongoing double-masked, randomized Phase II LUNA trial demonstrated similar outcomes with maintained BCVA and central subfield thickness, an 88-to-92-percent reduced treatment burden, and 54-to-69 percent of patients remaining injection-free at 52 weeks.11
Despite some cases of ocular inflammation and hypotony that led to early termination of the Phase I INFINITY study in patients with diabetic macular edema,12 ixo-vec appears to be well-tolerated in patients with nAMD in OPTIC and LUNA, with no serious adverse events recorded. Additionally, LUNA participant survey data revealed a strong patient preference (more than 90 percent) for ixo-vec over previous nAMD treatments.11 Because aqueous levels of aflibercept appeared sufficient and stable at all vector doses tested in these trials, the pivotal program will likely use the lowest dose to maximize safety.
 |
Intravitreal 4D-150
Another intravitreal gene therapy candidate is 4D-150 (4D Molecular Therapeutics), which uses AAV.R100, a vector designed using directed evolution in nonhuman primates rather than mice. Presumably, this vector could provide greater retinal tropism in humans at lower doses.
With two angiogenic targets, 4D-150 consists of a dual-transgene, including a codon-optimized sequence encoding aflibercept in addition to microRNA targeting VEGF-C. Interim 24-week results from the PRISM Phase II trial of the 3×1010 vg/eye dose showed similar promise in maintaining durable functional and anatomic gains, with participants achieving an 89-percent reduction in the rate of annualized anti-VEGF injections. No serious adverse events or clinically significant inflammation were reported on a prophylactic corticosteroid regimen.13
Suprachoroidal ABBV-RGX-314
Suprachoroidal injections of triamcinolone have been proven safe and effective in the treatment of uveitic macular edema,14 and recent studies from our lab and others suggest that they may be effective for delivering AAV vectors for gene therapy as well.15-17 This novel mode of delivery has the advantage of being an in-office procedure when compared to subretinal delivery. It also may offer better retinal transduction and lower immunogenicity than intravitreal injections.
This novel approach using suprachoroidal microneedles has been applied to ABBV-RGX-314 for both nAMD and diabetic retinopathy. Interim results from 106 patients enrolled in the Phase II AAVIATE study of suprachoroidal ABBV-RGX-314 have shown stable BCVA and CST at six months. Like subretinal delivery, patients had an 80-percent reduction in annualized injections, and 50 percent were injection-free.
Adverse events through six months were mild or moderate, and included conjunctival hemorrhage, increased intraocular pressure, episcleritis and conjunctival hyperemia. Mild ocular inflammation reported with suprachoroidal ABBV-RGX-314 appears to resolve with topical corticosteroids.18
Bottom line
Gene therapy targeting angiogenic factors such as VEGF may be effective in managing complex retinal conditions, including AMD. Subretinal, intravitreal and suprachoroidal AAV delivery all show durable gene expression and reduced anti-VEGF injection burden for patients with AMD.
The safety profile of these treatments varies by dose and administration route, with some ocular inflammation that appears to be prevented with local corticosteroid prophylaxis and some cases of retinal pigmentary changes. Additionally, postmarket reports of similar chorioretinal atrophy following subretinal administration of voretigene neparvovec RPE65 are limited, with functional outcomes overall remaining stable due to foveal sparing in most patients. It’s unclear whether these pigmentary changes are surgically driven, a result of direct vector toxicity or an inflammatory response.19-20 Although the prospect of a one-time therapy for nAMD is exciting, physicians will have to weigh the benefits against the risk of surgery or need for long-term corticosteroid use. RS
REFERENCES
1. Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related macular degeneration: A review. JAMA. 2024;331:147–157.
2. Brown MM, Brown GC, Stein JD, Roth Z, Campanella J, Beauchamp GR. Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol. 2005;40:277-287.
3. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, Phase 3 trial. Lancet. 2017;390:849-860.
4. Constable IJ, Pierce CM, Lai CM, et al. Phase 2a randomized clinical trial: Safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168-175.
5. Campochiaro PA, Avery R, Brown DM, et al. Gene therapy for neovascular age-related macular degeneration by subretinal delivery of RGX-314: A Phase 1/2a dose-escalation study. Lancet. 2024;403:1563-1573.
6. A randomized, partially masked, controlled, Phase 3 clinical study to evaluate the efficacy and safety of RGX-314 gene therapy in participants with nAMD (ASCENT). Clinicaltrials.gov identifier: NCT05407636. Updated October 16, 2024. Accessed February 26, 2025. https://clinicaltrials.gov/study/NCT05407636.
7. A randomized, partially masked, controlled, Phase 2b/3 clinical study to evaluate the efficacy and safety of RGX-314 gene therapy in participants with nAMD (ATMOSPHERE). Clinicaltrials.gov identifier: NCT04704921. Updated January 29, 2025. Accessed February 26, 2025. https://clinicaltrials.gov/study/NCT04704921.
8. Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra76
9. Grishanin R, Vuillemenot B, Sharma P, et al. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol Ther. 2019;27:118-129.
10. Khanani AM, Boyer DS, Wykoff CC, et al. Safety and efficacy of ixoberogene soroparvovec in neovascular age-related macular degeneration in the United States (OPTIC): A prospective, two-year, multicentre phase 1 study. EClinicalMedicine. 2023;67:102394.
11. Adverum biotechnologies announces positive 52-week Luna and 4-year optic results, and provides key pivotal program design elements [press release]. Redwood City, CA; Adverum Biotechnologies; November 18, 2024. Accessed February 26, 2025. https://investors.adverum.com/press_releases/news-details/2024/Adverum-Biotechnologies-Announces-Positive-52-Week-LUNA-and-4-Year-OPTIC-Results-and-Provides-Key-Pivotal-Program-Design-Elements .
12. A Phase 2, multi-center, randomized, double-masked, active controlled study of ADVM-022 (AAV.7m8-aflibercept) in subjects with diabetic macular edema [INFINITY]. Clinicaltrials.gov identifier: NCT04418427. Updated August 8, 2023. Accessed February 26, 2025. https://clinicaltrials.gov/study/NCT04418427.
13. 4DMT announces positive Phase 2 PRISM interim results for intravitreal 4D-150 in a broad wet AMD population affirming favorable safety profile and robust clinical activity [press release]. Emeryville, CA; Molecular Therapeutics; July 17, 2024. Accessed February 26, 2025. https://4dmt.gcs-web.com/news-releases/news-release-details/4dmt-announces-positive-phase-2-prism-interim-results .
14. Yeh S, Khurana RN, Shah M, et al; PEACHTREE study investigators. efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: Phase 3 randomized trial. Ophthalmology. 2020;127:948-955.
15. Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol Ther Methods Clin Dev. 2020;16:179-191.
16. Chung SH, Mollhoff IN, Mishra A, et al. Host immune responses after suprachoroidal delivery of AAV8 in nonhuman primate eyes. Hum Gene Ther. 2021;32:682-693.
17. Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019;129:4901-4911.
18. RegenXBio announces positive interim data from phase II AAVIATE trial of ABBV-RGX-314 for the treatment of wet AMD using suprachoroidal delivery [press release]. Rockville, MD; RegenXBio; January 16, 2024. Accessed February 26, 2025. https://ir.regenxbio.com/news-releases/news-release-details/regenxbio-announces-positive-interim-data-phase-ii-aaviater.
19. Gange WS, Sisk RA, Besirli CG, et al. Perifoveal chorioretinal atrophy after subretinal voretigene neparvovec-rzyl for RPE65-mediated Leber congenital amaurosis. Ophthalmol Retina. 2022;6:58-64.
20. Reichel FF, Seitz I, Wozar F, et al. Development of retinal atrophy after subretinal gene therapy with voretigene neparvovec. Br J Ophthalmol. 2023;107:1331-1335.



