|
|
Bio Dr. Zafar is a vitreoretinal surgery fellow at Wills Eye Hospital/MidAtlantic Retina in Philadelphia. She may be reached at: Disclosures: None |
An 86-year-old female was referred to our clinic for three weeks of floaters in her right eye associated with mild ocular discomfort. Past medical history was notable for hypertension, hyperlipidemia and pre-diabetes.
Work-up and imaging
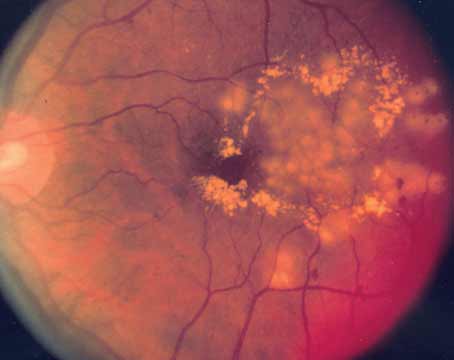
On exam, visual acuity was 20/30 OU. Intraocular pressure was normal, and no relative afferent pupillary defect was noted. Anterior segment exam OD was notable for mild conjunctival injection, fine corneal keratic precipitates and 3+ anterior chamber cell. Anterior segment OS was WNL. The patient was pseudophakic. Dilated fundus exam showed vitreous opacities OD with an inferotemporal area of retinal whitening (Figure 1). DFE OS was unremarkable. Given concern for infectious retinitis, the patient was sent to the ER for bloodwork and for intravitreal foscarnet. Oral valacyclovir and sulfamethoxazole and trimethoprim were also started to cover for viral retinitis and toxoplasmosis. A targeted systemic work-up was unremarkable including CBC, CMP, HIV, syphilis, tuberculosis and toxoplasma IgM/IgG. Aqueous fluid PCR testing was negative for toxoplasmosis, herpes simplex virus 1 and 2 (HSV) and varicella zoster (VZV) but returned positive for cytomegalovirus (CMV) (340,000 copies/ml).
The patient was subsequently diagnosed with CMV retinitis. Her valacyclovir was stopped and switched to valganciclovir 900 mg twice daily.
Additional work-up and follow-up
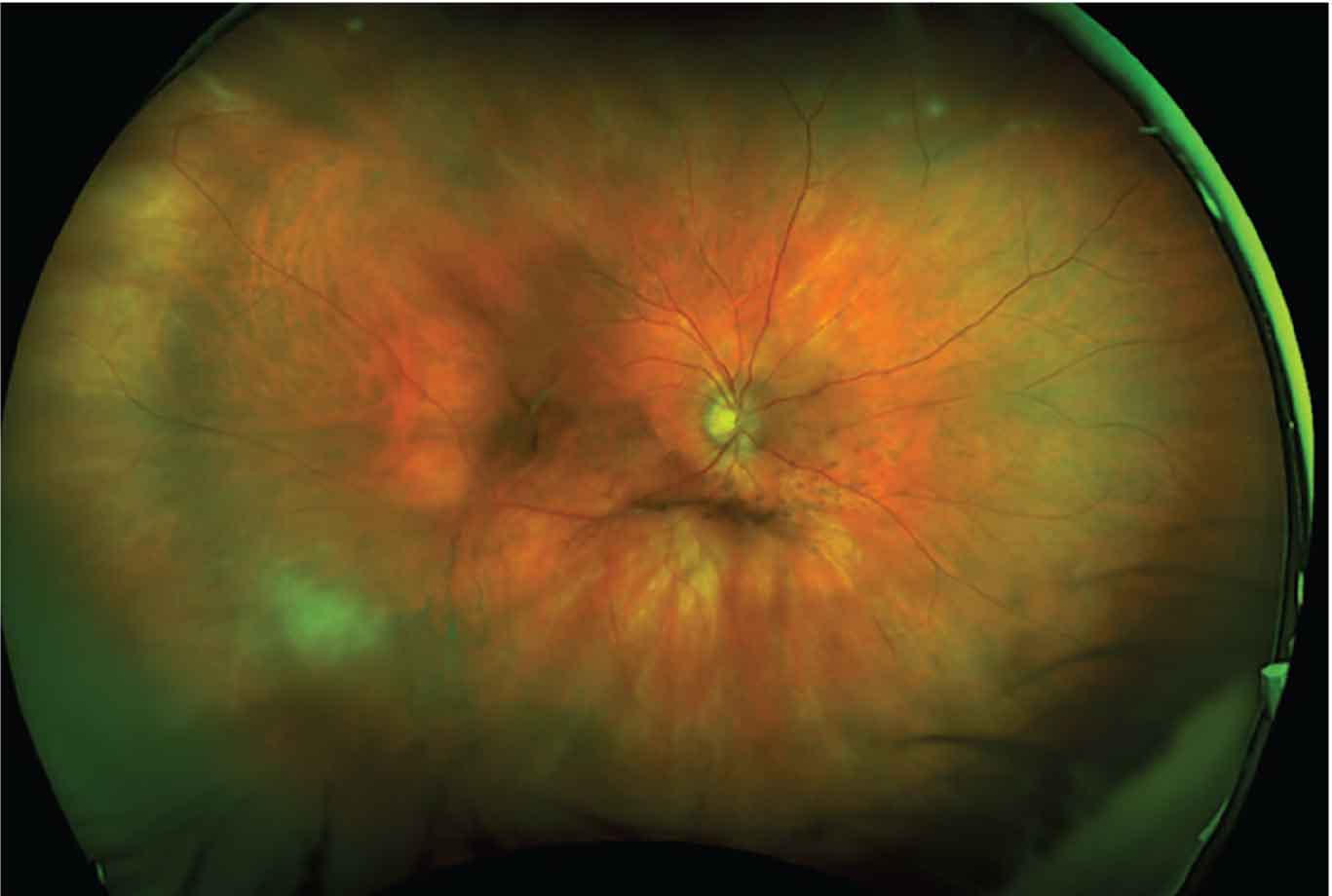
The patient’s absolute CD4+ and CD8+ cell counts were within normal limits. She was referred to her PCP for age-appropriate cancer screening which was unrevealing. Over the next six weeks, the patient responded well to therapy with complete resolution of the CMV lesion (Figure 2).
 |
|
Figure 1. Ultra-widefield fundus photograph of the right eye demonstrates vitreous opacities overlying the posterior pole and white retinal lesion in the retinal periphery inferotemporally. |
Discussion
CMV retinitis is classically a disease of the immunocompromised. Populations most at risk include those with HIV/AIDS, as well as bone marrow and solid organ transplant recipients.1 Clinically, CMV retinitis lesions often begin in the periphery and may appear either as areas of whitening with associated hemorrhage, variable small dot-like lesions (granular type) or, rarely, retinal vasculitis with perivascular sheathing.1
Although very rare, few cases of CMV retinitis occurring in immunocompetent patients have been reported in the literature. In most cases, subclinical immunosuppression related to old age and/or diabetes was hypothesized to increase the risk for CMV retinitis. In a review of 19 immunocompetent individuals with CMV retinitis, the mean patient age was 67 years and over half of all patients had diabetes.2,345
Cases of CMV retinitis in immunocompetent patients have also been reported after intravitreal or sub-Tenon’s steroid injections. One study found an overall incidence of 0.41 percent of viral retinitis following 736 intravitreal triamcinolone injections.6 The mean time from injection to the development of viral retinitis was 16 weeks. All three patients had an underlying immune-altering medical condition including DM, HIV or malignancy. In a second study, researchers identified 30 cases of viral retinitis following local corticosteroid administration.7 CMV was the most common etiology (23/30, 76.7 percent), followed by HSV (5/30, 16.7 percent) and VZV (1/30, 3.3 percent). The mean time from steroid administration to diagnosis of retinitis was 3.9 months for intravitreal injections and 1.8 months for sub-Tenon’s injection. Of the patients who developed CMV retinitis, 37 percent had diabetes and a mean age of 69.3 years.
 |
|
Figure 2. Ultra-widefield fundus photograph demonstrating resolution of CMV retinitis lesion on follow-up six weeks later |
Currently available therapies for CMV retinitis include intravenous and intravitreal ganciclovir and foscarnet, intravenous cidofovir and oral valganciclovir.1 A sustained-release intravitreal ganciclovir implant (Vitrasert) was approved in 1995 but was discontinued by the manufacturer in 2013 because of decreasing demand. In patients with AIDS, CMV therapy can be stopped if CD4 counts remain greater than 100 to 150 for three to six months after resolution of the retinitis. However, in patients who aren’t clinically immunosuppressed, no guidelines exist to guide the duration of therapy. Our patient responded well to intravitreal foscarnet and systemic valganciclovir. The valganciclovir was tapered to once daily following resolution of active retinitis. Her next follow-up visit is planned in another four weeks.
The Bottom Line
Although very uncommon, CMV retinitis can develop in older or diabetic patients who are seemingly immunocompetent. A prompt and thorough work-up is essential in these cases to rule out any underlying immunosuppression. RS
References
1. Port AD, Orlin A, Kiss S, et al. Cytomegalovirus retinitis: A review. J Ocul Pharmacol Ther. 2017; 33:224–234.
2. Tabatabaei SA, Cheraqpour K, Pour EK, Bohrani Sefidan B. Long-term prophylaxis in an immunocompetent patient with Cytomegalovirus retinitis: A case report and review of literature. J Ophthalmic Inflamm Infect. 2020;10:1:16.
3. Gupta S, Vemulakonda GA, Suhler EB, et al. Cytomegalovirus retinitis in the absence of AIDS. Canadian Journal of Ophthalmology. 2013;48:2:126-129.
4. Shukla R, Mishra AK, Verma A, Garg P, et al. A rare case of Cytomegalovirus retinitis in a young immunocompetent patient. Cureus. 2023;15:9:e44948.
5. Radwan A, Metzinger JL, Hinkle DM, Foster C. S. Cytomegalovirus retinitis in immunocompetent patients: Case reports and literature review. Ocul Immunol Inflamm. 2013;21:4:324-8.
6. Shah AM, et al. Viral retinitis after intravitreal triamcinolone injection in patients with predisposing medical comorbidities. Am J Ophthalmol. 2010;149:433–440.
7. Takakura A. et al. Viral retinitis following intraocular or periocular corticosteroid administration: A case series and comprehensive review of the literature. Ocul Immunol Inflamm. 2014;22:3:175-82.