Take-home points
|
 |
Bios Dr. Delaney (Jarocki) is a PGY-3 ophthalmology resident at the Cleveland Clinic Cole Eye Institute. Dr. Delaney will be pursuing a fellowship in vitreoretinal surgery. Dr. Sharma is an vitreoretinal surgeon and uveitis specialist at the Cleveland Clinic’s Cole Eye Institute, with a specific research interest in ocular imaging. He also serves as the Medical Director in the Information Technology Division for Ophthalmology, as the Uveitis Fellowship Director and the medical student clerkship director. He's an assistant professor of ophthalmology at the Cleveland Clinic Lerner College of Medicine. DISCLOSURES: Dr. Sharma consults and does contracted research for Genentech/Roche, and also does research for Acelyrin. Dr. Delaney (Jarocki) has no financial interest in any material presented. |
Noninfectious uveitis remains a significant challenge to treat due to its severity, chronicity and high recurrence rate. While the mainstay of initial uveitis treatment is corticosteroids, the well-known side effects of corticosteroids including systemic side effects and local side effects such as cataract and glaucoma limit their use. Various novel treatments in the pipeline for NIU include small molecule inhibitors, biologics and combination therapies.
Here, we will describe some of the emerging treatments.
Background
Uveitis describes ocular inflammation of the uveal tract, which includes the choroid, ciliary body and iris. The prevalence of uveitis in the United States is estimated to be 121 to 540 per 100,000 persons.1
In uveitis, immune cells such as T cells, B cells, macrophages and dendritic cells become activated and release cytokines such as TNF-α, IL-6, IL-1β and IFN-γ, which ultimately lead to intraocular inflammation.
Small Molecule Inhibitors
Brepocitinib, developed by Priovant Therapeutics, is an oral medication that selectively inhibits both Janus Kinase-1 (JAK1) and Tyrosine Kinase-2 (TYK2). JAK1 and TYK2 inhibition leads to downstream blockade of cytokines (IL-12 and IL-23), as well as modulation of Th1 and Th17 cell differentiation.2 The dual inhibition of JAK1 and TYK2-dependent downstream pathways is theorized to provide a greater immunomodulatory effect than JAK1 inhibition alone, while avoiding adverse events related to JAK2 and JAK3 inhibition such as infection.
A Phase II dose-ranging, randomized, double-masked trial (NEPTUNE) assessed oral brepocitinib in adults with NIU, excluding patients with anterior uveitis only.3 Twenty-six subjects with active NIU were randomized 2:1 to brepocitinib 45 mg or 15 mg once daily. All subjects received 60 mg/day oral prednisone upon entry for two weeks and were tapered over a six-week course. Participants were evaluated for treatment failure, a composite endpoint of ocular inflammation and visual acuity, as well as discontinuation of the medication or initiation of rescue therapy with corticosteroids. The study’s primary efficacy endpoint was the treatment failure rate at week 24, at which point patients had been off steroids for 16 weeks and only treated with brepocitinib.
At week 24, 29 percent of participants in the 45-mg group and 44 percent in the 15-mg group met treatment failure criteria, indicating lower rates of treatment failure compared to previous non-steroidal therapies for NIU such as adalimumab. Secondary endpoints, including improvements in vitreous haze grades, visual acuity and macular thickness, showed positive and dose-dependent results. Specifically, in the 45-mg group, 43 percent of those with baseline macular edema achieved resolution by week 24.
Brepocitinib demonstrates promise given its durable sustained response over 16 weeks without the use of steroids and relatively rapid action compared to studies evaluating adalimumab. The drug has been tested in more than 1,400 subjects in different inflammatory diseases, maintaining a safety profile similar to other JAK inhibitors, without additional safety signals identified. A Phase III trial for brepocitinib in NIU (CLARITY) is planned to commence in the second half of 2024.
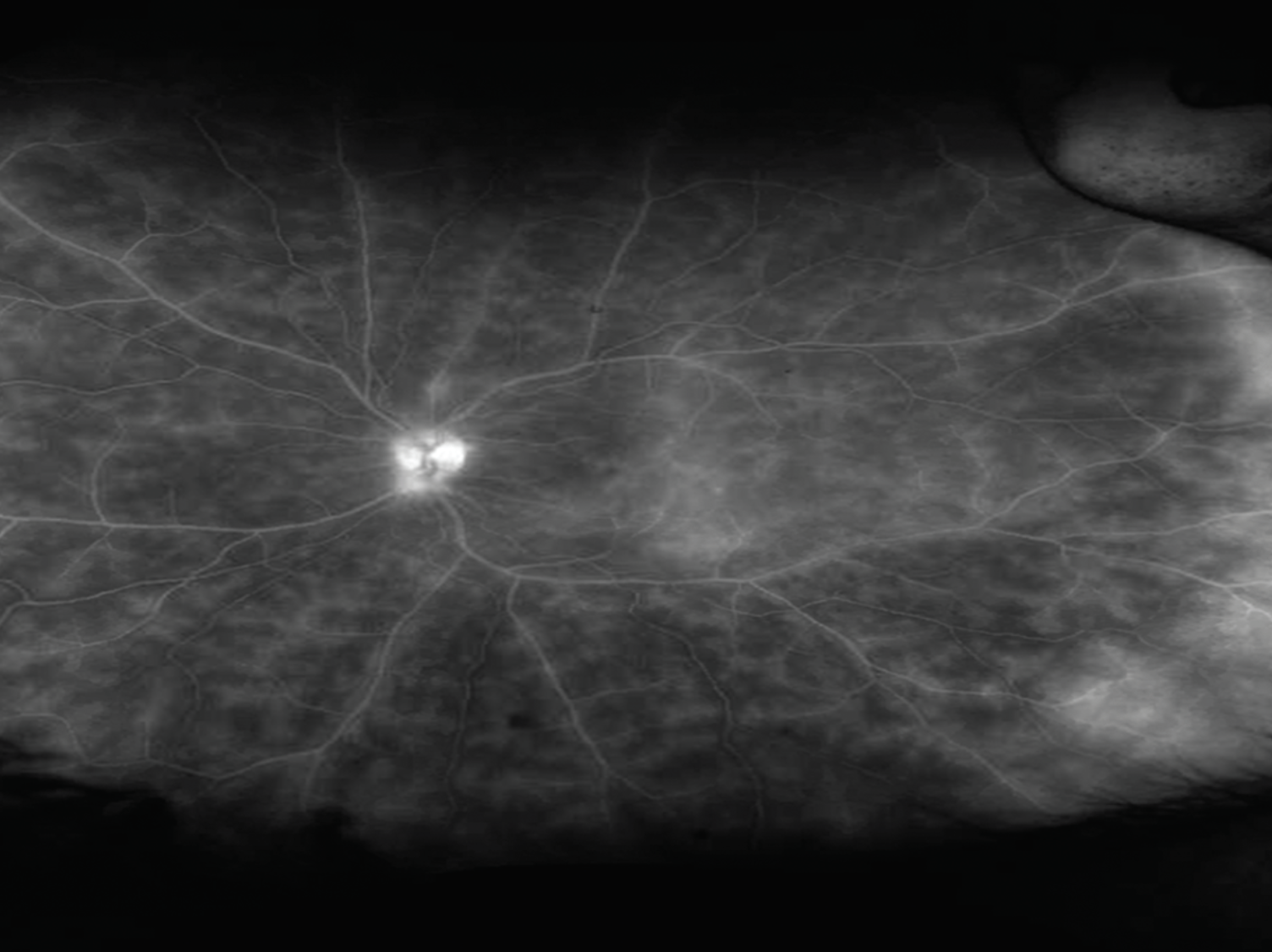 |
|
Fluorescein angiogram in a patient with intermediate uveitis showing extensive vascular leakage and capillary ferning. |
Biologics
There are a couple of biologics being studied for NIU treatment:
• Anti-IL-6. Vamikibart, RG6179/ RO7200220, developed by F. Hoffmann-La Roche, is an intravitreally delivered humanized anti–IL-6 monoclonal antibody. IL-6 plays a critical role in the differentiation of Th17 cells, which have been implicated in the pathogenesis of immune-mediated diseases.4 Elevated levels of IL-6 have also been observed in the vitreous of patients with uveitis.5 Tocilizumab, a systemically delivered anti-IL-6 medication has shown promising results in uveitis, so there is interest in local therapy to decrease systemic side effects.6
The DOVETAIL study, a Phase I trial of Vamikibart evaluated 37 participants with NIU and concurrent uveitic macular edema (UME), defined as central subfield thickness (CST) ≥325 µm on optical coherence tomography. Participants were randomized to three different doses of medication (0.25 mg, 1 mg and 2.5 mg) and monitored for changes in best-corrected visual acuity and macular thickness. Patients received three monthly intravitreal injections and were followed until week 36 (28 weeks post-treatment). BCVA improved by 9.3 ±1.6 letters and CST on OCT decreased by 161 ±28 μm at 12 weeks after three treatments, with these benefits maintained during the post-treatment observation period.7 Therefore, preliminary results suggest a potential benefit in managing UME associated with NIU.
There are currently two identical global, randomized, double masked, Phase III trials of RO7200220 (MEERKAT and SANDCAT) in patients with all forms of NIU and concurrent UME.8 Both trials will randomize patients into three arms: vamikibart 0.25 mg; vamikibart 1 mg; or sham control. Patients will be evaluated monthly over one year to assess the drug’s safety and effectiveness. The drug will be administered four times every four weeks through week 12, followed by as-needed dosing from week 20 through 48. The primary outcome measure will be the proportion of patients with ≥15 letter BCVA improvement at week 16 from baseline.
The results of these Phase III trials will be significant as they could potentially address the unmet need for effective nonsteroidal treatments. The current primary therapy for UME is steroids, which can have significant side effects such as increased intraocular pressure, cataracts and systemic health issues like hypertension and diabetes. Intravitreal anti-IL-6 if effective and safe would avoid the adverse effects of steroid treatment in patients with uveitis-associated macular edema.
• Anti-IL-17. Similar to IL-6, IL-17 is also an inflammatory cytokine that is produced by Th17 cells and recruits other inflammatory cytokines and chemokines like TNFα. IL-17 has also been found to be upregulated in systemic diseases associated with uveitis.9,10 There are currently multiple anti-IL-17 biologics being studied in auto-immune conditions. Secukinumab, developed by Novartis Pharmaceuticals, is a subcutaneously delivered IL-17 inhibitor that failed to meet the primary efficacy endpoints for uveitis treatment in multiple Phase III trials (SHIELD, INSURE and ENDURE). However, greater response rates with IV dosing of secukinumab suggests that patients may not have received sufficient drug with subcutaneous administration and that high-dose IV secukinumab may be necessary to deliver clinically therapeutic concentrations.11
This prompted the development of izokibep, a novel antibody fusion protein with an albumin binding domain to increase circulation time in the body. Izokibep (ABY-035) developed by Acelyrin is an anti-IL-17 medication whose molecular design is hypothesized to extend the drug’s half-life, improve tissue penetration, and increase target site drug concentration, in comparison to other anti-IL-17 formulations.12
Subcutaneous izokibep is currently being evaluated in NIU in a Phase IIb/III randomized, double masked, placebo-controlled trial. All participants will receive a standardized prednisone burst starting at 60 mg/day from day one to day 14, followed by a 13-week taper. Patients are required to have active disease despite the initial two-week steroid treatment for study inclusion. At the two-week mark, participants will be randomized to placebo or izokibep 160 mg. The study will evaluate time to treatment failure and is estimated to be completed in mid-2025.
Topical Anti-TNFα
While systemic anti-TNFα medications such as adalimumab have proven to be successful in NIU, serious adverse events including infections, myocardial infarctions, malignancies and hematologic reactions have been reported. There is hence a desire to develop an effective local version of this medication to avoid systemic side effects.
OCS-02 (Licaminlimab), developed by Oculis, is a new topical anti-TNFα antibody fragment. In a Phase II, multicenter, randomized, double-masked study, 43 adult patients with NIU with 2-3+ anterior chamber cell were randomized 3:1 to licaminlimab (60 mg/mL, eight drops/day for 15 days, four drops/day for seven days, then matching vehicle for seven days) or dexamethasone eye drops (eight drops/day for 15 days, tapering to one drop/day over 14 days).13 The primary endpoint was at least a two-step decrease in AC cell grade at day 15. At day 15, 56 percent of patients treated with licaminlimab had a treatment response. Of note, by day four, 36 percent of licaminlimab-treated patients were already responders. Dexamethasone response rate by day 15 was 90 percent, however the study wasn’t initially designed as a comparative study of the two treatment arms. Reassuringly, intraocular pressure wasn’t increased by licaminlimab during any point in the study. Licaminlimab is the first topical biologic demonstrated to have a treatment effect on NIU. There are currently no Phase III trials planned for this medication in NIU.
Topical and Intravitreal Dazdotuftide
Dazdotuftide manufactured by Tarsier Pharma is a small synthetic molecule that combines tuftsin and phosphorycholine. Both of these anti-inflammatory molecules have separately been shown to regulate the immune system and have a strong synergistic effect by inhibiting TLR-4, NRP-1 and ACE-2.14
Topical dazdotuftide, TRS01, has been studied in patients with anterior NIU, in a Phase III, global randomized, double-blinded, clinical trial (TRS4Vision). Patients were randomized 2:1 to TRS01 or prednisolone drops. The primary endpoint was anterior chamber cell at week four, where 48 percent of patients treated with TRS01 achieved no anterior segment cell vs. 68 percent of patients treated with prednisolone.15 TRS01 was inferior in proportion of patients reaching no cell at week four compared to prednisolone, the current standard of care. However, in a post-hoc analysis, two weeks after treatment had been completed, almost a third of responders treated with steroids had rebound of inflammation, while a higher rate of TRS01 responders, almost 90 percent, benefited from continued prolonged resolution of inflammation. This may indicate that individuals who initially respond to treatment with TRS01 may remain inactive for longer.
Notably, patients treated with TRS01 didn’t develop IOP elevation. Overall, there was a 5.3 times higher risk for developing elevated IOP with steroid treatment compared to TRS01. The average IOP per visit remained stable for patients treated with TRS01, while it increased for those treated with steroids. A post hoc analysis found that 13 percent of responders treated with steroids experienced a clinically meaningful IOP elevation (≥10 mmHg from initial), while none of the patients treated with TRS01 experienced such elevation. Therapies that are effective and don’t have an IOP effect are of critical interest in uveitis treatment.
A slow-release biodegradable intravitreal injection (TRS02) is also in development. No clinical trials have been announced for this formulation yet.
Bottom Line
In non-infectious uveitis treatment, critical unmet needs include local steroid-sparing medications and more effective systemic targeted therapeutics with better side-effect profiles. Several promising treatment options for NIU are now in various stages of development including oral small molecule inhibitors like brepocitinib, systemic therapeutics targeting IL-17 and intravitreal and topical biologics such as those targeting IL-6, TNFa antagonists, as well as combined pharmacologic agents like dazdotuftide. RS
REFERENCES
1. Zhang Y, Amin S, Lung KI, Seabury S, Rao N, Toy BC. Incidence, prevalence, and risk factors of infectious uveitis and scleritis in the United States: A claims-based analysis. PLoS One. 2020;15:8:e0237995.
2. Krueger JG, McInnes IB, Blauvelt A. Tyrosine kinase 2 and Janus kinase‒signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. Journal of the American Academy of Dermatology. 2022;86:1:148-157.
3. Roivant announces positive NEPTUNE study results for brepocitinib in non-infectious uveitis. Roivant. April 2, 2024. Accessed July 14, 2024. https://investor.roivant.com/news-releases/news-release-details/roivant-announces-positive-neptune-study-results-brepocitinib.
4. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:10:a016295.
5. Perez V, Papaliodis G, Chu D, Anzaar F, Christen W, Foster C. Elevated levels of interleukin 6 in the vitreous fluid of patients with pars planitis and posterior uveitis: The Massachusetts Eye & Ear experience and review of previous studies. Ocular Immunology and Inflammation 2004;12:3:205-214.
6. Sepah YJ, Sadiq MA, Chu DS, et al. Primary (month-6) outcomes of the STOP-Uveitis Study: Evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol. 2017;183:71-80. doi:10.1016/j.ajo.2017.08.019
7. Sharma S, Suhler E, Lin P, et al. A novel intravitreal anti-IL-6 monoclonal antibody for uveitic macular edema (UME): Preliminary results from the phase 1 DOVETAIL study. Investigative Ophthalmology & Visual Science 2023;64:8:5100.
8. Suhler EB, Steeples L, Elze M, Macgregor L, Silverman D, Haskova Z. IL-6 inhibition with vamikibart in patients with uveitic macular edema: phase 3 MEERKAT and SANDCAT trials. Investigative Ophthalmology & Visual Science. 2024;65:7:2609.
9. Guedes MCE, Borrego LM, Proença RD. Roles of interleukin-17 in uveitis. Indian Journal of Ophthalmology. 2016;64:9:628. doi:10.4103/0301-4738.194339
10. .Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocular Immunology and Inflammation. 2013;21:6:434-439.
11. Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122:5:939-948.
12. Klint S, Feldwisch J, Gudmundsdotter L, et al. Izokibep: Preclinical development and first-in-human study of a novel IL-17A neutralizing affibody molecule in patients with plaque psoriasis. mAbs. 2023;15:1:2209920.
13. Pasquali TA, Toyos MM, Abrams DB, Scales DK, Seaman JW, Weissgerber G. Topical ocular anti-tnfα agent licaminlimab in the treatment of acute anterior uveitis: A randomized phase ii pilot study. Transl Vis Sci Technol. 2022;11:6:14.
14. Shemer A, Kivity S, Shovman O, et al. Tuftsin-phosphorylcholine (TPC) equally effective to methylprednisolone in ameliorating lupus nephritis in a mice model. Clin Exp Immunol. 2018;193:2:160-166.
15. Tarsier Pharma announces results from a phase 3 clinical trial in subjects with noninfectious anterior uveitis including subjects with uveitic glaucoma. BioSpace. Accessed July 14, 2024. https://www.biospace.com/article/tarsier-pharma-announces-results-from-a-phase-3-clinical-trial-in-subjects-with-noninfectious-anterior-uveitis-including-subjects-with-uveitic-glaucoma/.



