 |
|
Bio Dr. Gupta is a PGY-3 ophthalmology resident at Rush University Medical Center. He will be pursuing a fellowship in vitreoretinal surgery. Dr. Raiji is a medical retina and uveitis specialist at Illinois Retina Associates and Rush University Medical Center. She is an associate professor of Ophthalmology at Rush University Medical College. Correspondence: |
Sarcoidosis is a complex, multisystem inflammatory disease with a myriad of clinical manifestations unified by the presence of noncaseating granulomas. Though approximately 30 to 60 percent of patients with diagnosed sarcoid develop ophthalmic manifestations, sarcoid uveitis is the most common manifestation, present in approximately 20 to 30 percent of these patients.1,2 This article will cover the diagnosis of sarcoid uveitis, including ophthalmic and systemic imaging, key lab findings and the current treatment approaches for effectively managing this condition.
 |
|
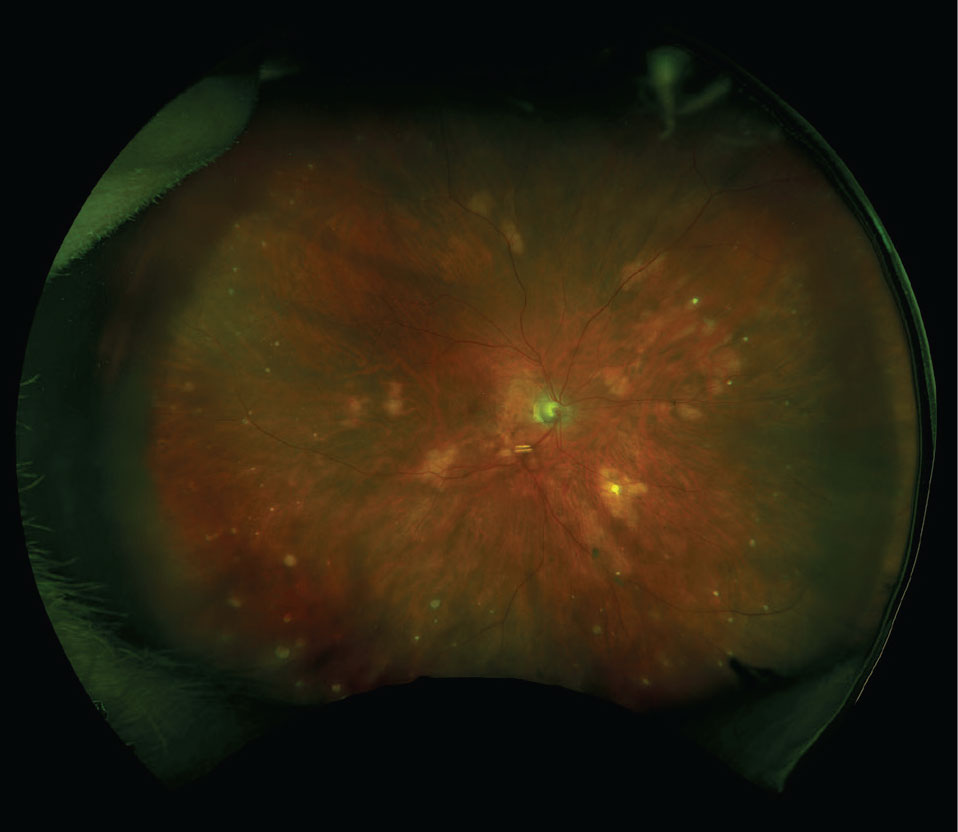
Figure 1. Fundus photograph of multifocal choroiditis with choroidal granulomas. |
Clinical Presentation
Sarcoid uveitis spans a wide spectrum of clinical manifestations. While anterior uveitis is the most common manifestation, the traditional characterization of granulomatous uveitis isn’t a pathognomonic or universal exam finding. Anterior signs may include, but aren’t limited to, keratic precipitates (classically in Arlt’s triangle), anterior and posterior synechiae, and iris nodules. Intermediate signs include vitritis, snowballs or snowbanks, and periphlebitis. Posterior findings are variable but classically include perivenous exudates resembling “candle-wax drippings,” non-occlusive retinal vasculitis, multifocal choroiditis and choroidal granulomas. Less common manifestations include retinal artery macroaneurysm (RAM), occlusive retinal vasculitis and optic nerve granulomas.3 Interestingly, retrospective studies have shown that while anterior uveitis may be more frequent in black patients, posterior uveitis may be more common in white patients. Additionally, chronic cases of posterior uveitis with complications may be more likely to present in white females with late-onset sarcoidosis.4,5
 |
|
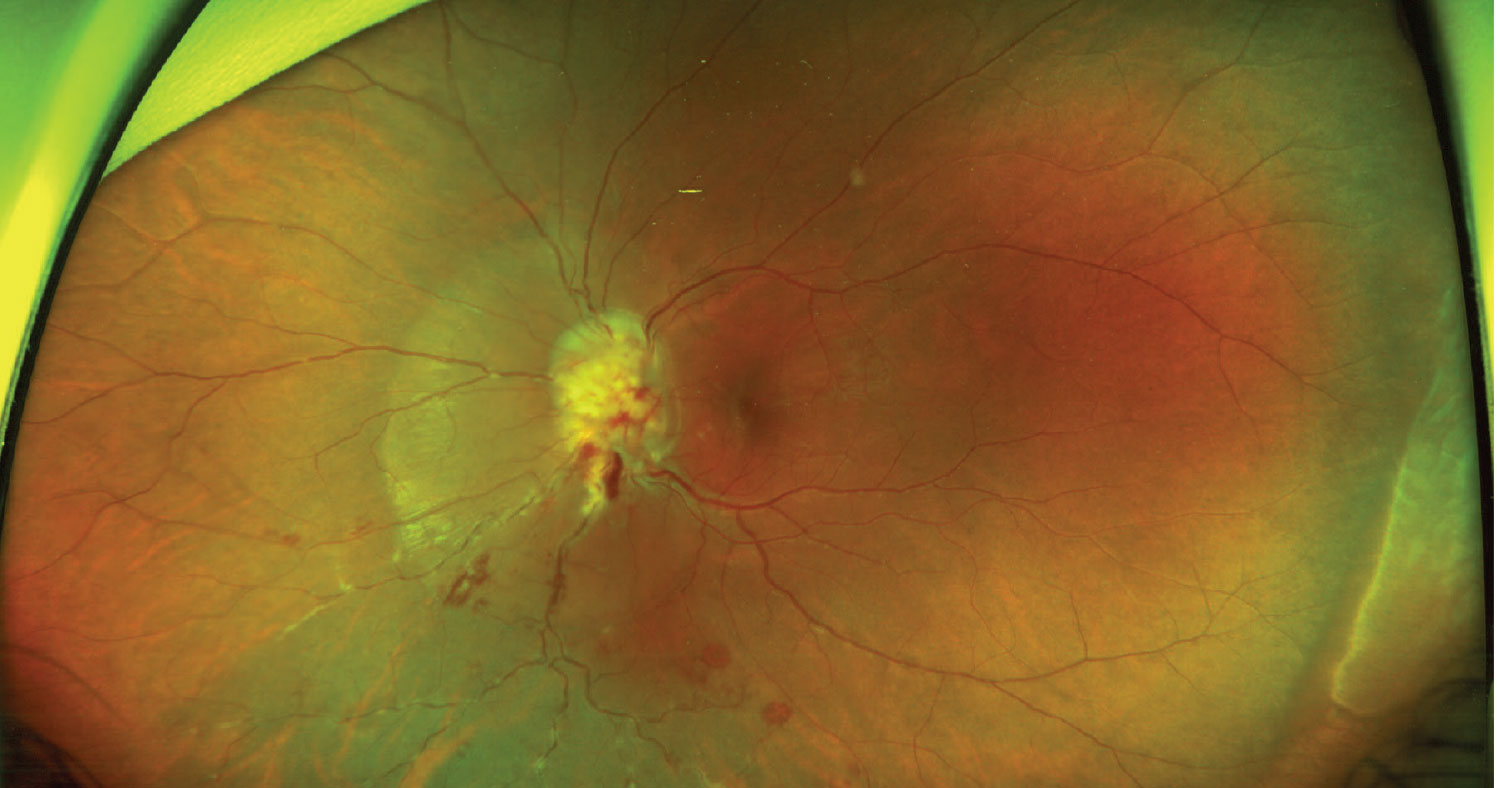
Figure 2. Fundus photograph of an optic nerve head granuloma with inferior retinal hemorrhages and perivascular “candle-wax drippings.” |
Diagnosis
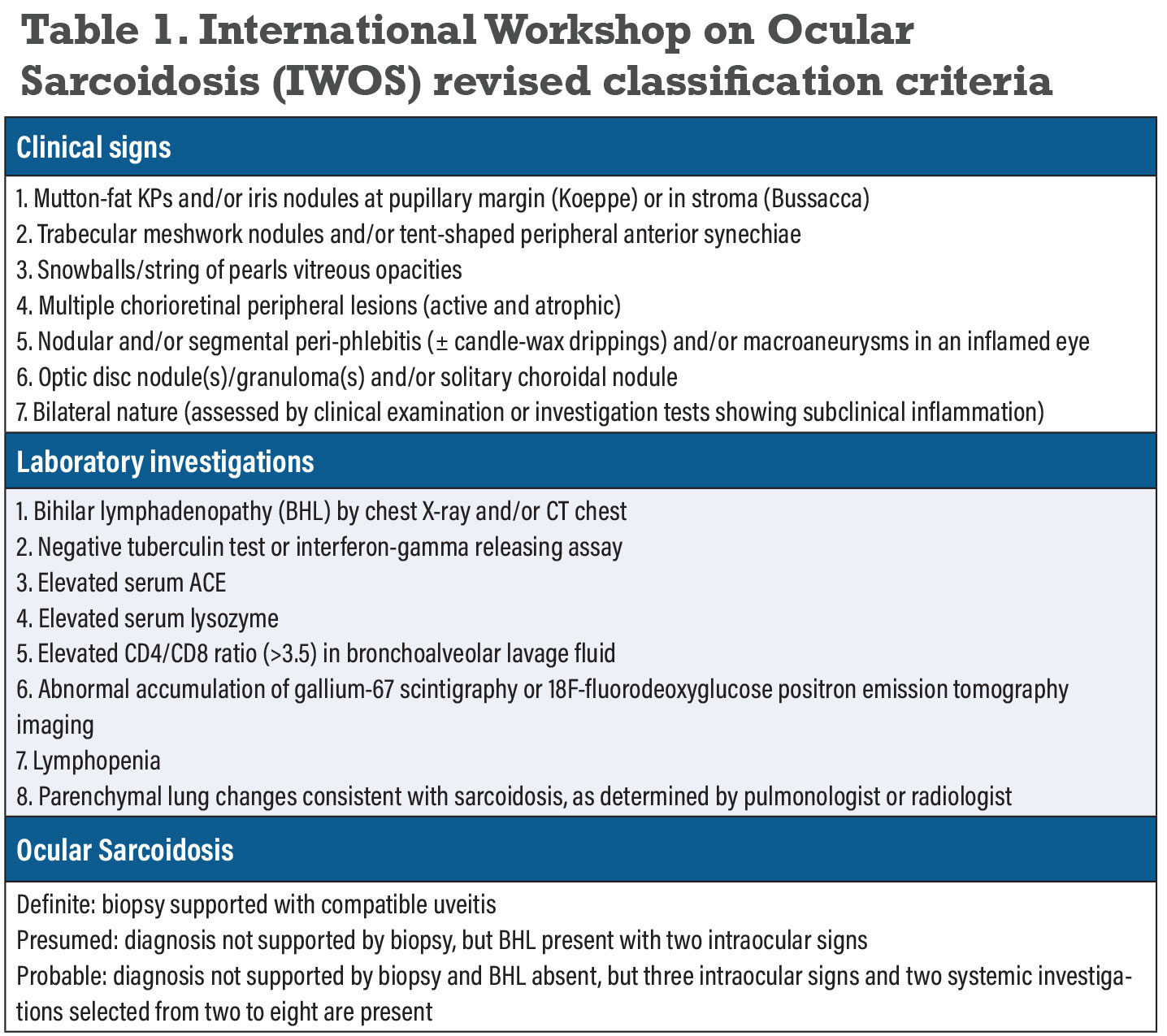
Since the spectrum of ocular inflammation is highly variable, diagnostic workups must be tailored to each patient. The presence of a compatible presentation, histologic evidence of non-caseating granuloma in any organ system and exclusion of other causes of granulomatous inflammation is sufficient to make a diagnosis. Conjunctival lesions, when present, are easily accessible for biopsy and should be examined closely. When biopsy isn’t available, alternative approaches to diagnosis may be used. The International Workshop on Ocular Sarcoidosis (IWOS) established criteria for the diagnosis of intraocular sarcoidosis in 2009 (later revised in 2019) when biopsy was either not performed or negative (Table 1).6
Ophthalmic Imaging
Fluorescein angiography is useful in detecting inflammatory breakdown of the blood-retinal barrier. Vasculitis will appear as staining or leakage of dye, predominantly affecting retinal veins, and may be diffuse or segmental.7 The arterial phase is crucial for detection of arterial inflammation that may lead to formation of RAMs. Findings such as perifoveal capillary disruption, hypo- or non-perfusion and accompanying small vessel disease such as microaneurysms or capillary dilatation can all be observed in active disease.7 The presence of optic disc leakage further indicates the presence of posterior segment inflammation. Choroidal granulomas may be scattered and typically demonstrate early hypofluorescence and late hyperfluorescence.7 Secondary complications such as choroidal neovascular membranes (CNVM) may also be evident angiographically.
 |
Indocyanine green angiography is useful for disease assessment given the predilection of sarcoid to affect the choroid. Active choroidal granulomas typically appear as dark lesions that are irregularly distributed and are hypocyanescent in the early and intermediate phases.8,9 ICGA is also beneficial when severe vitreous opacity limits FA findings.7,10 Combination of both ICGA and FA may be crucial to detection of subclinical inflammation.
Fundus photographs and autofluorescence images are useful in tracking clinically relevant findings. Sarcoid-associated choroidal granulomas are characteristically hyperautofluorescent.11
Enhanced-depth imaging optical coherence tomography (EDI-OCT) may be useful for detection of choroidal granulomas and tracking lesion size. Given its excellent characterization of the inner sclera and choroid, the sensitivity of choroidal granuloma detection on EDI-OCT may be higher or equivalent to ICGA, though the limitations of this technique confine visualization to the posterior pole.9
OCT angiography provides excellent vascular resolution for assessment of the outer retina and choroid. Choroidal granulomas are seen as dark lesions or defects in the vascular architecture.7 Choroidal flow voids correspond to the presence of choroidal granulomas and can be tracked during treatment to assess appropriate anatomic response. Secondary inflammatory CNVMs may also be well characterized by OCTA, as these have been found to reside near the edge of choroidal granulomas.12
Laboratory Findings
Serum Angiotensin Converting Enzyme (ACE) is elevated in granulomatous disease, typically correlating with disease activity. Although useful, its reliability decreases in younger individuals, patients taking ACE inhibitors and patients taking systemic corticosteroids.13,14 Lysozyme is also a marker of granulomatous inflammation, though it may be elevated in the presence of infectious uveitis.15
Serum soluble interleukin-2 receptor (sIL2R), a marker for T lymphocyte activation, can be used to measure disease severity and may distinguish between active and inactive sarcoidosis. However, its limited availability and lack of mention in the IWOS criteria limit its clinical utility.14,16
Systemic Imaging
Bilateral hilar lymphadenopathy is classically observed in systemic sarcoidosis and included in the IWOS criteria for diagnosis. While Chest X-Ray (CXR) is a useful first-line screening tool, the advent of high-resolution computerized tomography (HRCT) provides higher sensitivity and specificity for detection of pulmonary sarcoid.17 The use of HRCT in cases with high clinical suspicion may prove beneficial for timely diagnosis of systemic sarcoidosis.
Fluorine-18 fluorodeoxyglucose positron emission tomography (18-FDG-PET) may detect occult sites of sarcoidosis activity, highlighting possible targets for biopsy.18 Multiple recent studies have illustrated the higher sensitivity of 18-FDG-PET as compared to HRCT in cases with high clinical suspicion.19-21 Its cost and availability should be factored into its utility in clinical practice.
Arrhythmias from cardiac involvement are an important cause of mortality among patients with sarcoidosis. In patients with suspected sarcoidosis-related uveitis, an EKG should be performed to evaluate for any arrythmia.
Treatment
Corticosteroids are the typical first line treatment in sarcoid-associated uveitis. The IWOS indications for steroid therapy are listed as below:22
1. Anterior uveitis. Indications include anterior chamber cell, keratic precipitates, iris nodules, angle nodules, new posterior synechiae and elevated IOP with no alternative explanation.
2. Intermediate uveitis. Indolent intermediate uveitis doesn’t require treatment, but manifestations impacting vision such as diffuse vitreous opacities, snowball- or snowbank-like vitreous opacities or cystoid macular edema (CME) are indications for treatment.
3. Posterior uveitis. Optic disc nodules or granulomas, nodular and/or segmental periphlebitis, active chorioretinitis and CME warrant steroid therapy.
Local steroid therapy can be administered via topical, periocular, intravitreal or suprachoroidal modalities. Systemic treatment should be considered for patients with systemic involvement, severe intermediate and posterior uveitides, or severe anterior uveitis unresponsive to local therapy. The IWOS recommends 0.5 to 1 mg/kg/day of systemic prednisone with a maximum daily dose of 80 mg. This may be maintained for two to four weeks and tapered over subsequent months.22 If the disease is refractory to oral therapy, systemic pulse dose (IV methylprednisolone) may be considered in conjunction with a multidisciplinary medical team.
Cases refractory to high-dose steroids or those requiring long-term steroid therapy benefit from steroid-sparing immunosuppression.23,24 While relatively few patients with active sarcoidosis require steroid-sparing immunosuppression (compared with other noninfectious uveitides), treatment is generally effective.25 Conventional disease-modifying antirheumatic drugs (DMARDS) such as methotrexate, mycophenolate, azathioprine and cyclosporine are generally considered to be first-line steroid-sparing therapeutic agents.22
Third-line therapy is reserved for cases refractory to initial steroid sparing immunosuppression and involves biologic therapeutics. When initiating biologic therapy, further investigation is warranted to rule out the presence of alternative granulomatous conditions given the historical effectiveness of first-line therapies.22,23,26 Anti-tumor necrosis factor alpha agents such as adalimumab and infliximab have both demonstrated significant efficacy with favorable safety profiles in patients who’ve failed second-line therapies.27,28 Of note, some biologic therapies such as etanercept, adalimumab, abatacept, infliximab and golimumab have been implicated in inducing sarcoid-like uveitis and dermatologic sarcoid-like lesions in patients with or without an existing diagnosis of sarcoidosis.29,30
Though rarely required, newer biologics such as rituximab, tocilizumab and Janus Kinase (JAK) inhibitors are available for severe cases refractory to first-line biologic agents.31-33
Bottom line
Sarcoid uveitis is a well-documented but highly variable inflammatory condition that is vision-threatening. Given its potential to present as intraocular inflammation, ophthalmologists are on the forefront in the diagnosis and management of sarcoidosis and its ophthalmic manifestations. RS
REFERENCES
1. Jamilloux Y, Kodjikian L, Broussolle C, Sève P. Sarcoidosis and uveitis. Autoimmun Rev 2014;13:8:840-849.
2. Ma SP, Rogers SL, Hall AJ, et al. Sarcoidosis-related uveitis: Clinical presentation, disease course, and rates of systemic disease progression after uveitis diagnosis. Am J Ophthalmol 2019;198:30:36.
3. Samalia PD, Lim LL, Niederer RL. Insights into the diagnosis and management of sarcoid uveitis: A review. Clin Experiment Ophthalmol 2024;52:3:294-316.
4. Febvay C, Kodjikian L, Maucort-Boulch D, et al. Clinical features and diagnostic evaluation of 83 biopsy-proven sarcoid uveitis cases. Br J Ophthalmol 2015;99:10:1372-1376.
5. Rothova A, Alberts C, Glasius E, Kijlstra A, Buitenhuis HJ, Breebaart AC. Risk factors for ocular sarcoidosis. Doc Ophthalmol Adv Ophthalmol 1989;72:3-4:287-296.
6. Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol 2019;103:10:1418-1422.
7. Mahendradas P, Maruyama K, Mizuuchi K, Kawali A, Kitaichi N. Multimodal imaging in ocular sarcoidosis. Ocul Immunol Inflamm 2020;28:8:1205-1211.
8. Wolfensberger TJ, Herbort CP. Indocyanine green angiographic features in ocular sarcoidosis. Ophthalmology 1999;106:2:285-289.
9. Parrulli S, Invernizzi A, Monteduro D, et al. Longitudinal follow-up of choroidal granulomas with indocyanine green angiography and optical coherence tomography angiography: A lesion-based analysis. Retina Phila Pa 2022;42:5:906-914.
10. El Ameen A, Herbort CP. Comparison of retinal and choroidal involvement in sarcoidosis-related chorioretinitis using fluorescein and indocyanine green angiography. J Ophthalmic Vis Res 2018;13:4:426-432.
11. Baş Z, Sajjadi Z, Shields CL. Sarcoid granuloma of the choroid and ciliary body in 50 cases. Retina Phila Pa 2023;43:11:1842-1851.
12. Agarwal A, Invernizzi A, Singh RB, et al. An update on inflammatory choroidal neovascularization: Epidemiology, multimodal imaging, and management. J Ophthalmic Inflamm Infect 2018;8:1:13.
13. Niederer RL, Al-Janabi A, Lightman SL, Tomkins-Netzer O. Serum angiotensin-converting enzyme has a high negative predictive value in the investigation for systemic sarcoidosis. Am J Ophthalmol 2018;194:82-87.
14. Allegri P, Olivari S, Rissotto F, Rissotto R. Sarcoid uveitis: An intriguing challenger. Med Kaunas Lith 2022;58:7:898.
15. Sahin O, Ziaei A, Karaismailoğlu E, et al. The serum angiotensin converting enzyme and lysozyme levels in patients with ocular involvement of autoimmune and infectious diseases. BMC Ophthalmol 2016;16:19.
16. Vanmaris RMM, Rijkers GT. Biological role of the soluble interleukin-2 receptor in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG 2017;34:2:122-129.
17. Niederer RL, Sims JL. Utility of screening investigations for systemic sarcoidosis in undifferentiated uveitis. Am J Ophthalmol 2019;206:149-153.
18. Sève P, Jamilloux Y, Tilikete C, Gerfaud-Valentin M, Kodjikian L, El Jammal T. Ocular sarcoidosis. Semin Respir Crit Care Med 2020;41:5:673-688.
19. Chauvelot P, Skanjeti A, Jamilloux Y, et al. 18F-fluorodeoxyglucose positron emission tomography is useful for the diagnosis of intraocular sarcoidosis in patients with a normal CT scan. Br J Ophthalmol 2019;103:11:1650-1655.
20. Seve P, Billotey C, Janier M, Grange JD, Broussolle C, Kodjikian L. Fluorodeoxyglucose positron emission tomography for the diagnosis of sarcoidosis in patients with unexplained chronic uveitis. Ocul Immunol Inflamm 2009;17:3:179-184.
21. Rahmi A, Deshayes E, Maucort-Boulch D, et al. Intraocular sarcoidosis: Association of clinical characteristics of uveitis with findings from 18F-labelled fluorodeoxyglucose positron emission tomography. Br J Ophthalmol 2012;96:1:99-103.
22. Takase H, Acharya NR, Babu K, et al. Recommendations for the management of ocular sarcoidosis from the International Workshop on Ocular Sarcoidosis. Br J Ophthalmol 2021;105:11:1515-1519.
23. El Jammal T, Jamilloux Y, Gerfaud-Valentin M, Valeyre D, Sève P. Refractory sarcoidosis: A review. Ther Clin Risk Manag 2020;16:323-345.
24. Jabs DA. Immunosuppression for the uveitides. Ophthalmology 2018;125:2:193-202.
25. Niederer RL, Sharief L, Tomkins-Netzer O, Lightman SL. Uveitis in sarcoidosis - Clinical features and comparison with other non-infectious uveitis. Ocul Immunol Inflamm 2023;31:2:367-373.
26. Giorgiutti S, Jacquot R, El Jammal T, et al. Sarcoidosis-related uveitis: A review. J Clin Med 2023;12:9:3194.
27. Ogbue OD, Malhotra P, Akku R, Jayaprakash TP, Khan S. Biologic therapies in sarcoidosis and uveitis: A review. Cureus 2020;12:7:e9057.
28. Vallet H, Seve P, Biard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: A multicenter study from the French Uveitis Network. Arthritis Rheumatol Hoboken NJ 2016;68:6:1522-1530.
29. Decock A, Van Assche G, Vermeire S, Wuyts W, Ferrante M. Sarcoidosis-like lesions: Another paradoxical reaction to anti-TNF therapy? J Crohn’s Colitis 2017;11:3:378-383.
30. Sobolewska B, Baglivo E, Edwards AO, et al. Drug-induced sarcoid uveitis with biologics. Ocul Immunol Inflamm 2022;30:4:907-914.
31. Sepah YJ, Sadiq MA, Chu DS, et al. Primary (Month-6) outcomes of the STOP-Uveitis Study: Evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol 2017;183:71-80.
32. Deuter CME, Zierhut M, Igney-Oertel A, et al. Tocilizumab in uveitic macular edema refractory to previous immunomodulatory treatment. Ocul Immunol Inflamm 2017;25:2:215-220.
33. Lower EE, Baughman RP, Kaufman AH. Rituximab for refractory granulomatous eye disease. Clin Ophthalmol 2012;6:1613-1618.




