 |
Bios Dr. Fortenbach is an assistant professor of ophthalmology at the University of Washington, Seattle, where Dr. Chee is an associate professor of ophthalmology, Dr. Tummala is an ophthalmology resident, Dr. Bonnell a vitreoretinal surgery fellow and Dr. Yoda an assistant professor of pathology. DISCLOSURES: The authors have no relevant relationships to disclose. |
A 44-year-old male was referred to our tertiary care hospital for evaluation after experiencing bilateral floaters and blurry vision for five years. His ophthalmologist had previously diagnosed him with intermediate uveitis.
The patient had pars plana vitrectomy of the right eye one year before he came to our clinic, which resulted in improved vision and floaters for a few weeks, but then the symptoms worsened. Vitreous pathology following surgery showed small lymphocytes within a background of amorphous material.
He also received an injection of sub-Tenon’s kenalog in the left eye three months before presentation without a change in symptoms. A prior systemic work-up included testing for antineutrophil cytoplasmic antibody, antinuclear antibody, rheumatoid factor and serum protein electrophoresis, all of which were negative.
Examination findings
Examination showed best corrected visual acuity of 20/50 in the right eye and count fingers in the left eye. Pupils were equal and reactive without a relative afferent pupillary defect. Intraocular pressure was 16 mmHg in the right eye and 44 mmHg in the left.
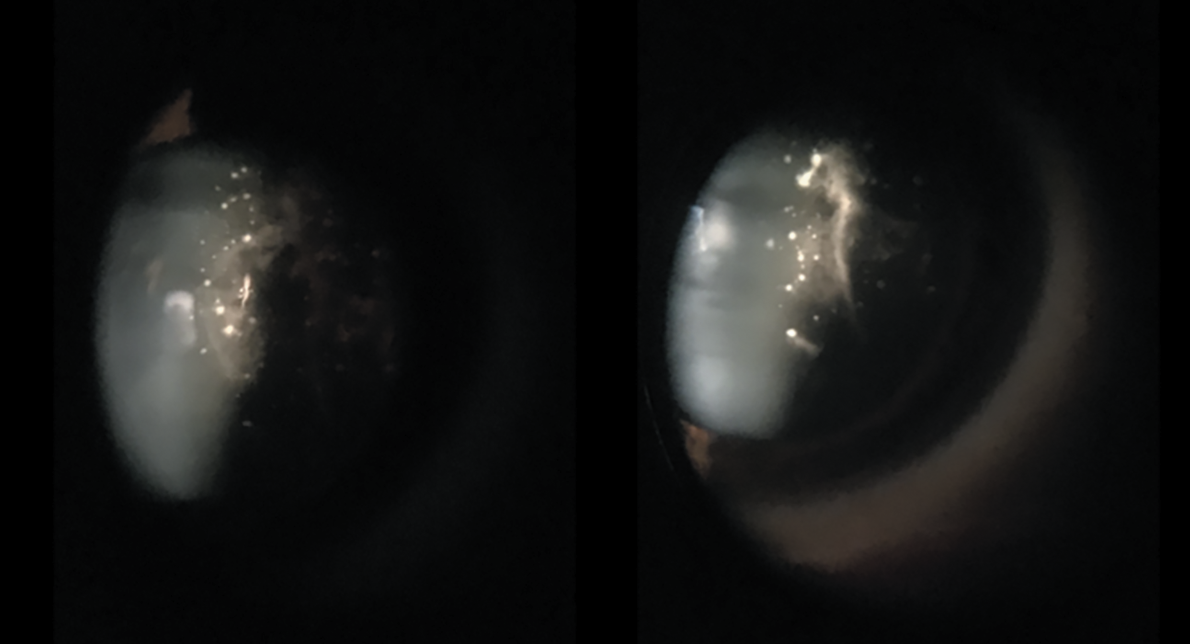
Slit lamp examination revealed a quiet anterior chamber. No keratic precipitates or posterior synechiae were evident. Both eyes had fluffy, white amorphous material stuck to the posterior lens capsule that extended into the anterior vitreous (Figure 1). The dilated fundus examination revealed white deposits along the blood vessels in both eyes, but was otherwise normal (Figure 2).
 |
|
Figure 1. White amorphous material attached to the posterior lens capsule of both eyes demonstrate “glass wool” appearance of the vitreous. |
Work-up
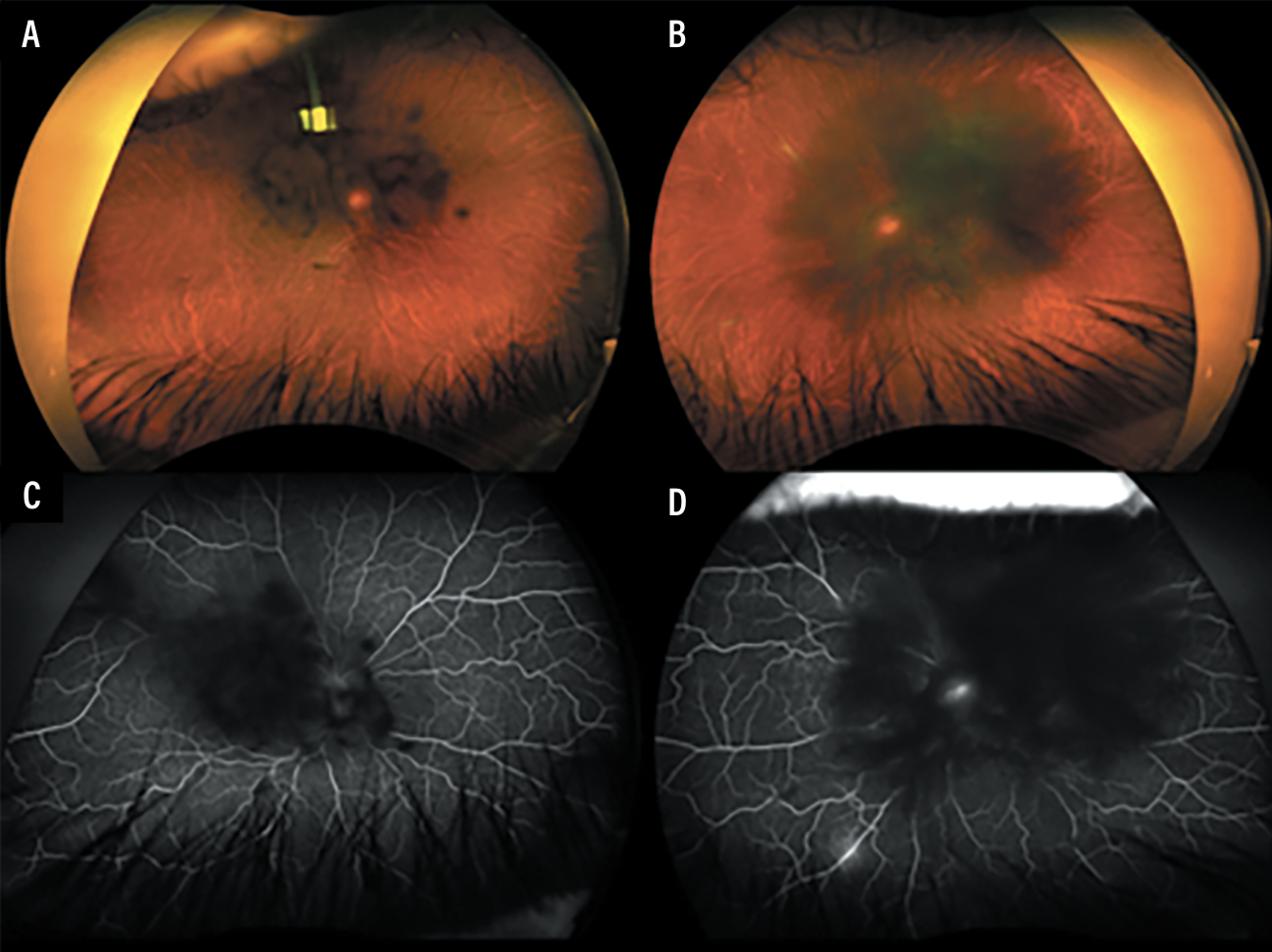
Fluorescein angiography demonstrated bilateral central blocking from the lenticular and vitreous opacities. The left eye had possible leakage from the optic nerve and late venous leakage inferonasally. Optical coherence tomography was limited by the lenticular and vitreous opacities.
 |
|
Figure 2. A, B) Fundus photography of the right and left eyes, respectively, demonstrate central vitreous opacities and white deposits along the peripheral blood vessels. C, D) Fluorescein angiography of the right and left eyes captured at around 5 minutes and 30 seconds, demonstrate central blockage from lenticular and vitreous opacities, possible nerve leakage of the left eye and inferior venous leakage. |
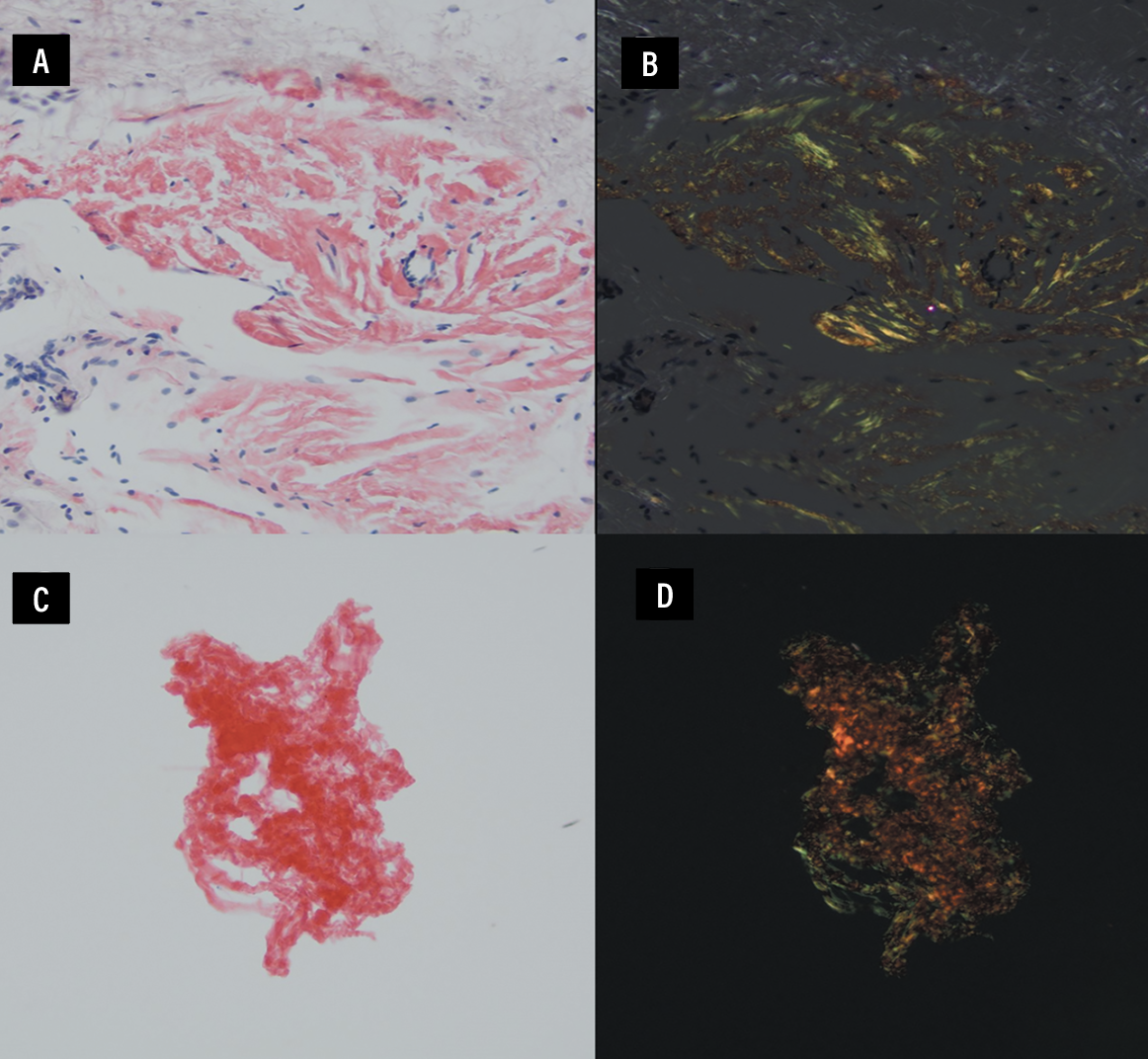
The patient then underwent a diagnostic PPV and conjunctival biopsy of the left eye, which showed Congo red staining of proteinaceous material with apple green birefringence (Figure 3). Liquid chromatography tandem mass spectrometry performed on the conjunctival biopsy revealed a peptide profile consistent with transthyretin type amyloid.
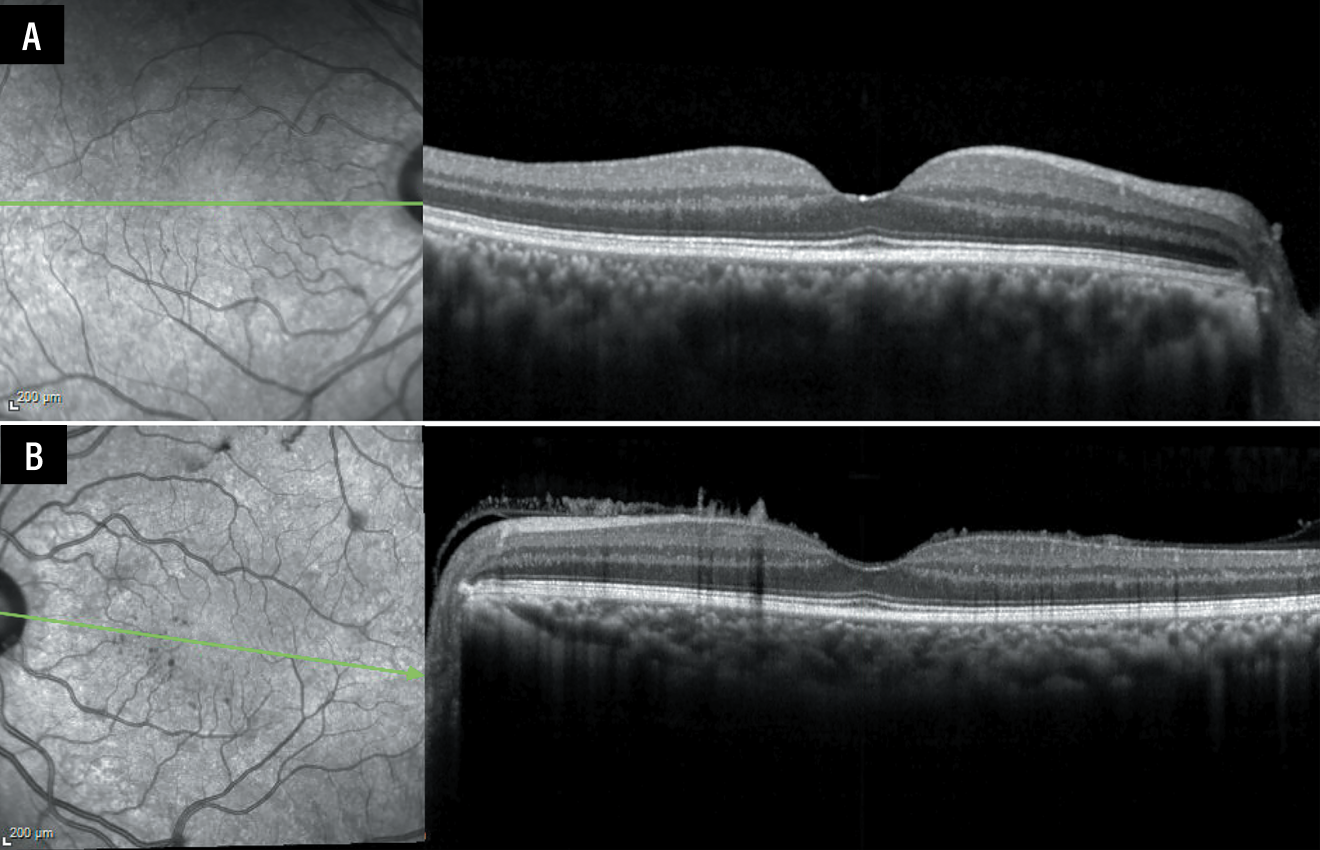
At the time of surgery, the hyaloid could not be lifted. Optical coherence tomography following surgery revealed needle-shaped projections extending from the retinal surface into the posterior hyaloid of the left eye, characteristic of ocular amyloidosis (Figure 4).1
 |
|
Figure 3. A, B) Congo red and apple green birefringence, respectively, of conjunctival tissue at 200x magnification. C, D) Congo red and apple green birefringence of the vitreous ThinPrep sample at 200x magnification. |
Diagnosis and Management
Genetic testing showed the patient to be a carrier of a pathogenic variant of the transthyretin (ATTRv) gene. Our patient was diagnosed with transthyretin amyloidosis. Elevated IOP in the left eye required insertion of an Ahmed glaucoma valve. Ocular hypertension and glaucoma can be seen in ocular amyloidosis because of the amyloid deposits in the trabecular meshwork.2
The patient then underwent a repeat PPV with membrane peel and cataract
surgery in the left eye, and is awaiting a repeat PPV and cataract surgery in the right eye. At his most recent follow-up, BCVA was 20/50 in the right eye, 20/80 in the left, and IOP remained controlled.
Following vitrectomy, he was referred to cardiology and neurology for evaluation. He was found to have cardiac amyloidosis and amyloid-related polyneuropathy. He’s being followed closely for these conditions.
 |
|
Figure 4. A) Optical coherence tomography of the right eye was unremarkable. B) Imaging of the left eye after pars plana vitrectomy revealed classic needle-shaped deposits on the anterior retinal surface extending into the anterior vitreous. |
Discussion
Amyloidosis refers to a rare and diverse group of diseases characterized by the extracellular deposition of misfolded and insoluble proteins within multiple organ systems. The most common form of systemic amyloidosis is light-chain amyloidosis, followed by transthyretin and secondary amyloidosis.3
Ocular involvement varies with phenotype and depends on the amyloidosis subtype. Currently 36 proteins have been associated with amyloidosis. Of these, transthyretin, gelsolin, keratoepithelin and lactoferrin are known to have ocular involvement.4
Transthyretin amyloidosis is an autosomal dominant disease with variable expressivity. Approximately 10 percent of patients with the ATTRv genotype present with ocular involvement.5 Transthyretin is mainly produced in the liver, but it’s also synthesized in the retinal pigment epithelium and the choroid plexuses of the brain.6
Ocular involvement generally occurs later in the disease course and is unrelated to the extent of systemic symptoms.7 Amyloid deposits have been found throughout the eye and in the lacrimal gland. Ophthalmic manifestations include vitreous opacities, retinal and choroidal vascular changes, secondary open-angle glaucoma, abnormal conjunctival vessels, pupillary changes, keratoconjunctivitis sicca, optic neuropathy and neurotrophic corneal ulcers secondary to corneal nerve damage. 4
Vitreous involvement in ATTRv amyloidosis is usually bilateral and asymmetric. The vitreous collagen fibers act as a scaffold for transthyretin amyloid aggregation. Vitreous amyloidosis is thought to be secondary to the production of abnormal transthyretin by the RPE.7 PPV is the current standard of treatment to remove vitreous amyloid and improve visual acuity. Relapsing vitreous amyloidosis often requires repeat PPV. 4
Retinal and choroidal vessels can also have amyloid deposition, causing peripheral retinal ischemia. It’s important to monitor the peripheral retina and choroid with fluorescein angiography and indocyanine green angiography in patients with longstanding disease to detect areas of ischemia and initiate therapy with panretinal photocoagulation, if necessary.4,8,9
Diagnosis of transthyretin amyloidosis is made by the identification of amyloid deposits in a tissue sample and genetic testing. ATTRv often has a delayed diagnosis because of its diverse clinical presentation.
Floaters from vitreous amyloidosis can be the presenting sign in some patients, requiring a high index of suspicion by ophthalmologists.10 Our case demonstrates the classic “glass wool” appearance of the vitreous, which may tip a retina specialist off to this unique disease. The needle-shaped deposits on the anterior retinal surface are also a characteristic of ocular amyloidosis.
Following diagnosis, patients with amyloidosis should be promptly referred for further systemic workup and consideration of systemic treatments, which include transthyretin stabilizers, RNA-targeted therapies and liver transplantation.11 Ocular synthesis of transthyretin persists following liver transplant. Patients have been shown to have progressive ocular manifestations of their disease and require continued ophthalmologic follow-up.7,12
Bottom line
The retina specialist may be the first to diagnose amyloidosis, a rare, heterogeneous group of diseases that can be fatal. But it’s a great masquerader, so diagnosis is often delayed. Observation of characteristic findings, such as the classic “glass wool” appearance of the vitreous and needle-shaped retinal deposits on OCT, can help raise clinical suspicion. Congo red staining of a vitreous sample may reveal proteinaceous material with apple green birefringence. Prompt referral for systemic evaluation and consideration of systemic treatment is essential. RS
References
1. Tasiopoulou A, Ong D, Lightman S. Characteristic needle-shaped pattern seen on OCT in a patient with ocular amyloidosis. Ophthalmol Retina. 2021;5:99-101.
2. Nelson GA, Edward DP, Wilensky JT. Ocular amyloidosis and secondary glaucoma. Ophthalmology. 1999;106:1363-1366.
3. Reynolds MM, Veverka KK, Gertz MA, et al. Ocular manifestations of systemic amyloidosis. Retina. 2018;38:1371-1376.
4. Minnella AM, Rissotto R, Antoniazzi E, et al. Ocular involvement in hereditary amyloidosis. Genes. 2021;12:955.
5. Dammacco R, Merlini G, Lisch W, et al. Amyloidosis and ocular involvement: An overview. Semin Ophthalmol. 2019;35, 7–26
6. Martone RL, Herbert J, Dwork A, Schon EA. Transthyretin is synthesized in the mammalian eye. Biochem Biophys Res Commun. 1988;151:905–912.
7. Martins AC, Rosa AM, Costa E, et al. Ocular manifestations and therapeutic options in patients with familial amyloid polyneuropathy: A systematic review. Biomed Res Int. 2015;2015:282405.
8. Reynolds MM, Veverka KK, Gertz MA, et al. Ocular manifestations of familial transthyretin amyloidosis. Am J Ophthalmol. 2017;183:156–162.
9. Kitahara J, Yoshinaga T, Kakihara S, et al. Ocular findings in patients with acquired ATTRv amyloidosis following domino liver transplantation. PLoS One. 2023;18:e0291716.
10. Ong DE, Davis JT, O’Day WT, Bok D. Synthesis and secretion of retinol-binding protein and transthyretin by cultured retinal pigment epithelium. Biochemistry. 1994;33:1835–1842.
11. Ferreira N. Ophthalmologic changes in transthyretin familial amyloid polyneuropathy (ATTR-FAP). Orphanet J Rare Dis. 2015;10(Suppl 1):19.
12. Sandgren O, Kjellgren D, Suhr OB. Ocular manifestations in liver transplant recipients with familial amyloid polyneuropathy. Acta Ophthalmol. 2008;86:520–524.



