Take-home points
|
 |
Bios Dr. Hua is an assistant professor and Dr. Berrocal is a professor of clinical ophthalmology at Bascom Palmer Eye Institute, University of Miami. Both specialize in pediatric and adult retina and vitreous diseases. DISCLOSURES: The authors have no relevant financial disclosures. |
The gene therapy pipeline for inherited retinal degenerations has exploded in the past decade. To date, voretigene neparvovec (Luxturna, Spark Therapeutics) is the only Food and Drug Administration-approved gene therapy for an IRD. Luxturna specifically treats patients with biallelic RPE65 mutation-associated retinal dystrophy. But in the IRD treatment pipeline, many investigative therapies don’t rely on mutation-specific gene replacement.
In the umbrella of gene therapies for IRDs, two subcategories exist (Figure): supplementation/replacement, such as voretigene neparvovec; and neuroprotection.1
Neuroprotection aims to restore photosensitivity by creating new photosensors and coupling them to the remaining retinal circuitry. This is independent of the specific gene mutation and depends only on the structural and functional state of the retinal circuit.1 In the subcategory of neuroprotection, three different strategies exist: optogenetics; stem cell approaches; and electronic implants, such as the Argus II (Cortigent).
Here, we’ll focus on optogenetics and provide a framework for understanding this gene therapy approach, discuss translational barriers, and review optogenetic therapies in the pipeline.
Optogenetics: A brief history
Optogenetics dates to 1971, when the German biochemist Dieter Oesterhelt, PhD, discovered bacteriorhodopsin—a microbial opsin, or light-sensitive membrane protein, in Halobacteria.2
Decades later in 2002, Dr. Oesterhelt’s protegee, Peter Hegemann, PhD, discovered channelrhodopsin (ChR), a light-activated transmembrane protein that could serve as a tool for “measuring and/or manipulating electrical gradients across cell membranes.”3
Subsequently in 2005, Karl Deisseroth, MD, PhD, Georg Nagel, PhD, and collaborators showed that light could induce action potentials in cultured nerve cells expressing channelrhodopsin-2 (ChR2).4
The next step would be to translate ex vivo expression of light-sensitive microbial opsins to in vivo neural circuitry in mice. In a landmark study in 2006, Anding Bi, PhD, and colleagues successfully used the viral vector, adeno-associated virus (AAV), delivered via intravitreal injection, to express ChR2 in retinal ganglion cells in mice.5
The retinitis pigmentosa (rd1/rd1) model mice with absent photoreceptors demonstrated light-evoked responses and expression of light-activated ChR2 compared to no response and expression in control mice. This study laid the groundwork for optogenetic gene therapy in retinal degenerations.
What is optogenetics?
Optogenetics uses gene therapy to precisely control existing neural circuitry through light-sensitive proteins, or opsins. For cellular context, bipolar cells and RGCs remain intact even in advanced stages of IRDs.6 Optogenetics can be used to leverage existing bipolar cells or RGCs to express opsins which bypass photoreceptors or even generate “artificial” receptors from ganglion cells.1 Most commonly, AAV is used to express and insert opsins such as ChR2 into retinal neurons.
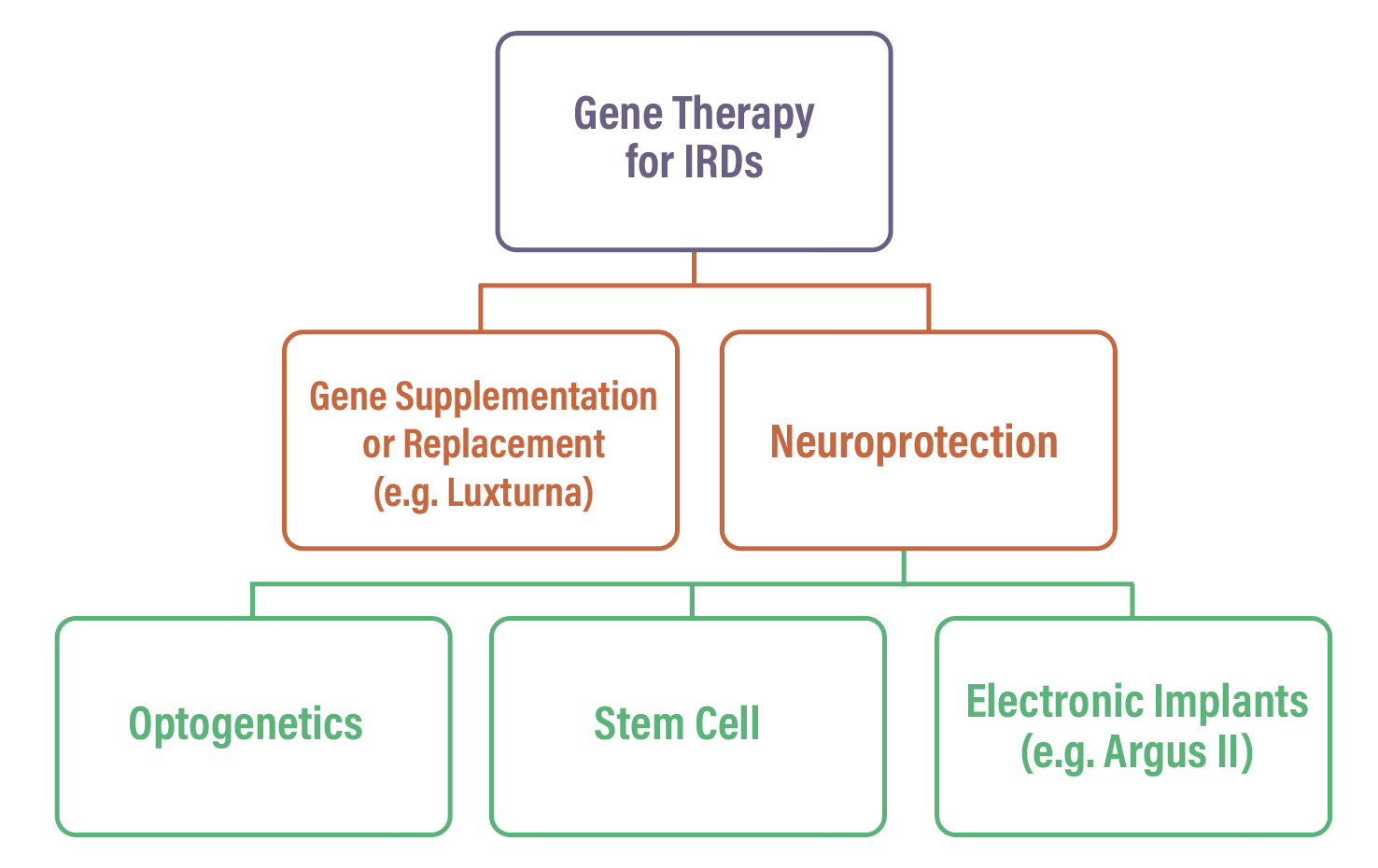 |
| The different categories of gene therapy for inherited retinal diseases. |
Barriers to clinical translation
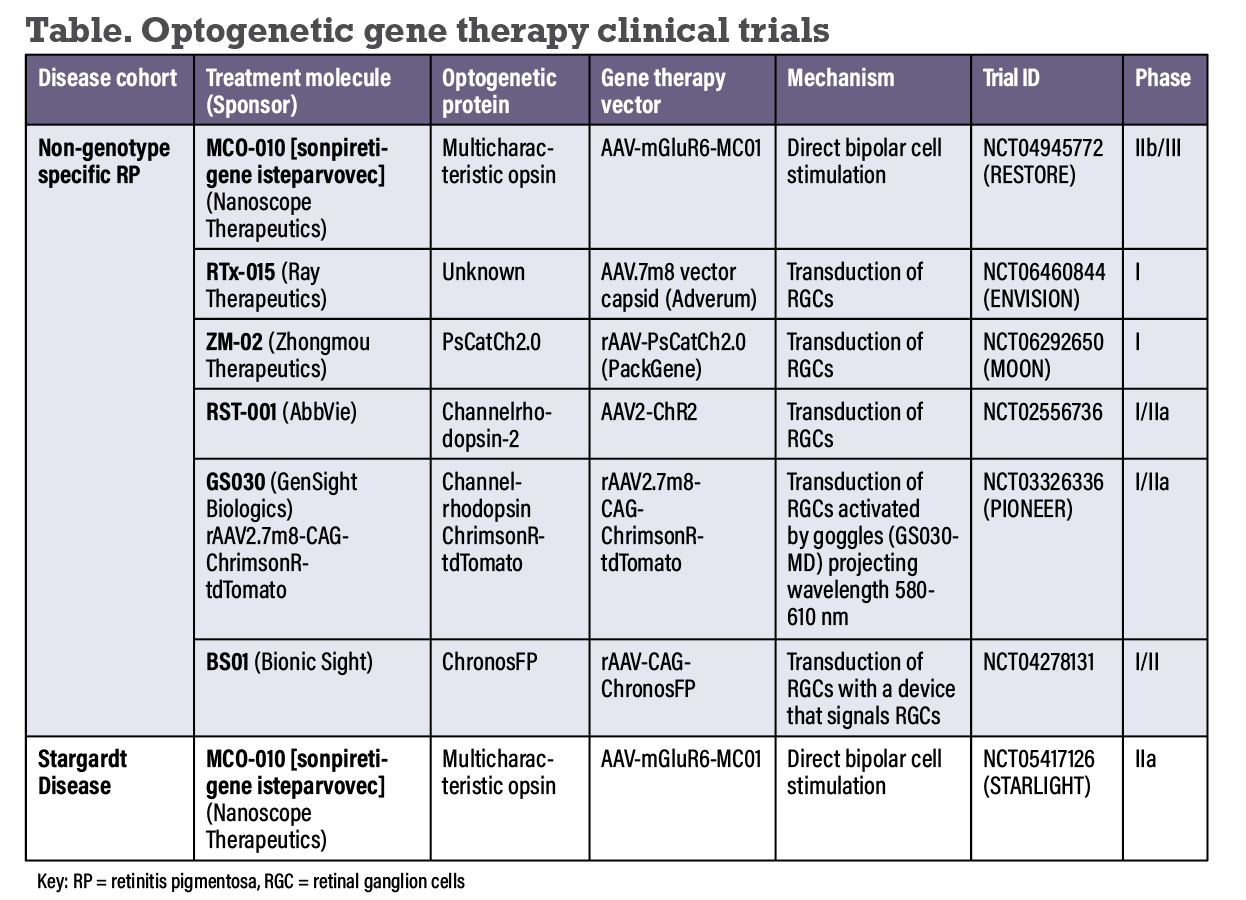
Currently, optogenetic sensors respond to a narrow range of light intensities, limiting its practical and clinical applications. Solutions include the development of increased dynamic range of optogenetically transformed cells or the use of an external device that amplifies light and/or acquires images across a large range of intensities.1,6 Two Phase I/II clinical trials with the optogenetic gene therapies GS030 (GenSight Biologics) and BS01 (Bionic Sight) require the use of an external device (Table below).7,8
Existing optogenetic approaches depend on specific light wavelengths to activate opsins. At this time, no optogenetic approaches allow for color perception.1
Concern also exists that certain opsins, such as ChR, require stimulation with shorter wavelengths, such as blue light, have greater potential for phototoxicity.6 As a result, there is a push for red-shifted opsins that minimize thermal effects and oxidative stress and decrease phototoxicity.
Immunogenicity always presents challenges with gene therapy. Future therapies would require prescreening of patients for particular serotypes of AAV antibodies that could neutralize gene therapy delivery. Additionally, uncontrolled eye movements in low-vision patients could interfere with image projection in the regions around optogenetically transduced areas of the retina.1
The unique mosaic anatomy of the fovea and structural remodeling of retinal neurons, gliosis and disorganization of the retinal layers can also scatter or absorb incoming light, limiting the restoration of vision.1,6 Photophobia in patients with inherited retinal diseases and pupillary constriction in the blue light spectrum may present biological barriers to the delivery of light to opsins transduced in the retina.1
 |
Optogenetic therapies in the pipeline
Since the seminal study by Dr. Bi and colleagues demonstrated proof of concept of retinal optogenetic gene therapy, a number of different light-activated microbial and animal opsins have been developed. Here, we provide a summary of optogenetic gene therapies in the IRD pipeline. All are delivered via intravitreal injection.
• Sonpiretigene isteparvovec (MCO-010, Nanoscope) uses the viral vector AAV-mGluR6-MC01 and promoter-enhancer mGluR6 to target bipolar cells to express a multicharacteristic opsin (MCO) therapy in bipolar cells.9 MCO is designed to be activatable under ambient light, expanding the potential of this therapy beyond high-intensity light conditions.10,11
The Phase IIb/III RESTORE study enrolled 28 patients with advanced retinitis pigmentosa in a multicenter randomized, double-masked, sham-controlled dose-escalation protocol.12 Patients had severe vision loss—most had hand motion, light perception, or worse.
Topline results showed that high-dose MCO-010 resulted in an improved BCVA of 20/1,500 and 20/900 at weeks 52 and 76, and the low-dose group had an improved BCVA of 20/1,300 at those time points.13 The study included a three-week oral steroid taper that started as prophylaxis three days before treatment. Twelve of 18 patients had intraocular inflammation, which was controlled with topical steroids. According to Nanoscope, high-dose MCO-010 (1.211 gc/eye) is the planned commercial dose. The company plans to file a biologics license application this year.
 |
The STARLIGHT trial was a Phase II multicenter, open-label, single-dose study of MCO-010 in patients with a clinical diagnosis of Stargardt disease.14 Topline results showed a mean BCVA improvement of 5.5 letters, with a 15-letter improvement with a wearable magnifier. No serious adverse effects were observed.15 The company plans to move forward with a Phase III trial.
• RTx-015 (Ray Therapeutics) targets RGCs to transduce a proprietary opsin delivered with a AAV.7m8 vector capsid.16 In the Phase I, open-label, nonrandomized, dose-escalation study, nine patients with RP are to be enrolled in up to three dose cohorts of RTx-015.17
• ZM-02 (Zhongmou Therapeutics) is a novel engineered ChR variant opsin, PsCatCh2.0, that requires 100-fold less light intensity than its predecessor.18 It’s delivered with an AAV vector (provided by PackGene) targeting RGCs.18,19 China-based Zhongmou is conducting a Phase I dose-escalating trial (MOON) of ZM-02 in RP patients.20
• RST-001 (AbbVie) encodes for opsin ChR2 targeting the RGCs. The Phase I/IIa trial was an open-label, dose-escalation study in patients with advanced RP.21 In 14 patients, no serious adverse events were recorded. Four intraocular inflammatory events were reported (anterior chamber cell, iritis, vitreal cells, vitritis), and an optic neuritis event was reported.
• GS030 (GenSight Biologics) is a recombinant rAAV2 vector containing optimized channelrhodopsin ChrimsonR-tdTomato. Patients with end-stage RP use a medical device called GS030-MD for light stimulation after receiving the therapy. Topline results of PIONEER, the Phase I/IIa open-label, nonrandomized, dose-escalation study showed improvement from bare LP vision to locating and counting objects.7 Seventy percent of patients had IOI, which responded to corticosteroid treatment and resolved without sequelae in all patients.
• BS01 (Bionic Sight) encodes for chronosFP, an opsin reported to be 10 times more sensitive to light than ChR2 and has a longer excitation wavelength, reducing the risk of phototoxicity.22 BS01 is the focus of a Phase I/II nonrandomized, open-label dose escalation study of a nonreplicating, rep/cap-deleted recombinant AAV vector expressing chronosFP in patients with RP.23
After an intravitreal injection of the gene therapy, patients wear a device that relays signals to the RGCs. Preliminary data showed that 10 of 11 patients had improved BCVA, with seven showing measurable increase on the ETDRS chart.24 No serious adverse events were recorded. The FDA this year granted Regenerative Medicine Advanced Therapy designation for BS01.
Bottom line
Optogenetics is a gene-agnostic approach to therapy for degenerative retinal diseases. Light-sensitive proteins called opsins are delivered via AAV vectors and typically transduced via bipolar cells or retinal ganglion cells to bypass dysfunctional photoreceptors. Investigative optogenetic gene therapies are in various phases of clinical trials. RS
REFERENCES
1. Sahel JA, Roska B. Gene therapy for blindness. Annu Rev Neurosci. 2013;36:467-488.
2. Friedman JM. How the discovery of microbial opsins led to the development of optogenetics. Cell. 2021;184:5687-5689.
3. Nagel G, Ollig D, Fuhrmann M, et al. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296:2395-2398.
4. Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18:1213-1225.
5. Bi A, Cui J, Ma YP, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23-33.
6. Poboży K, Poboży T, Domański P, Derczyński M, Konarski W, Domańska-Poboża J. Evolution of Light-sensitive proteins in optogenetic approaches for vision restoration: A comprehensive review. Biomedicines. 2025;13:429.
7. GenSight Biologics announces 1 year safety data and efficacy signals from PIONEER Phase I/II clinical trial of GS030, an optogenetic treatment candidate for retinitis pigmentosa [press release]. Paris, France; GenSight Biologics; February 13, 2023. Accessed March 2, 2025. https://www.gensight-biologics.com/2023/02/13/gensight-biologics-announces-1-year-safety-data-and-efficacy-signals-from-pioneer-phase-i-ii-clinical-trial-of-gs030-an-optogenetic-treatment-candidate-for-retinitis-pigmentosa/.
8. Bionic Sight reports meaningful vision improvements for RP patients receiving highest dose of its emerging optogenetic therapy. Foundation Fighting Blindness; April 21, 2023. Accessed March 1, 2025. https://www.fightingblindness.org/news/bionic-sight-reports-meaningful-vision-improvements-for-rp-patients-receiving-highest-dose-of-its-emerging-optogenetic-therapy-860.
9. Nanoscope Therapeutics announces positive top-line results from randomized controlled trial of MCO-010 for retinitis pigmentosa. Dallas, TX; Nanoscope Therapeutics; March 26, 2024. Accessed March 4, 2025. https://nanostherapeutics.com/2024/03/26/nanoscope-therapeutics-announces-top-line-results-from-ph2-trial-of-mco-010-for-retinitis-pigmentosa/.
10. Wright W, Gajjeraman S, Batabyal S, et al. Restoring vision in mice with retinal degeneration using multicharacteristic opsin. Neurophotonics. 2017;4:041412.
11. Wright W, Pradhan S, Bhattacharya S, et al. Multi-characteristic opsin enabled vision restoration. Proc. SPIE 10052. Optogenetics and Optical Manipulation. February 8, 2017; 100520Q vol 10052.
12. Efficacy and safety of MCO-010 optogenetic therapy in adults with retinitis pigmentosa (RESTORE). National Clinical Trial Identifier NCT 04945772. Updated March 22, 2024. https://clinicaltrials.gov/study/NCT04945772?term=NCT04945772&rank=1.
13. Singer M. BCVA analysis of low- or high-dose MCO-010 optogenetic therapy for retinitis pigmentosa. Paper presented at American Society of Retina Specialists annual meeting. July 19, 2024; Stockholm, Sweden.
14. Safety and effects of a single intravitreal injection of vMCO-010 optogenetic therapy in subjects with Stargardt disease (STARLIGHT). National Clinical Trials Identifier NCT05417126. Updated November 9, 2023. https://clinicaltrials.gov/study/NCT05417126?term=NCT05417126&rank=1.
15. Tsang SH. MCO-010 optogenetic therapy for vision loss in Stargardt disease: Topline data from the phase 2 STARLIGHT trial. Paper presented at American Academy of Ophthalmology annual meeting. November 3-6, 2023; San Francisco, CA.
16. Adverum Biotechnologies grants license to Ray Therapeutics Inc. for the AAV.7m8 intravitreal capsid [press release]. Redwood City, CA; Adverum Biotechnologies; June 12,2023. Accessed 2 Mar 2025. https://investors.adverum.com/press_releases/news-details/2023/Adverum-Biotechnologies-Grants-License-to-Ray-Therapeutics-Inc.-for-the-AAV.7m8-Intravitreal-Capsid/default.aspx.
17. Study to evaluate safety of RTx-015 injection in retinitis pigmentosa or choroideremia patients (ENVISION). Updated March 25, 2025. National Clinical Trials Identifier NCT06460844. https://clinicaltrials.gov/study/NCT06460844?term=NCT06460844&rank=1.
18. Chen F, Duan X, Yu Y, et al. Visual function restoration with a highly sensitive and fast Channelrhodopsin in blind mice. Signal Transduct Target Ther. 2022;7:104.
19. Zhongmou Therapeutics unveils promising clinical data for innovative retinitis pigmentosa gene therapy. PackGene; April 1, 2024. Accessed March 2, 2025. https://www.packgene.com/frontier/240401/.
20. Safety and efficacy study of novel gene therapy ZM-02 for retinitis pigmentosa patients (MOON). Updated December 13, 2024. National Clinical Trials Identifier NCT06292650. https://clinicaltrials.gov/study/NCT06292650?term=NCT06292650&rank=1.
21. RST-001 Phase I/II trial for advanced retinitis pigmentosa. Updated November 8, 2024. National Clinical Trials Identifier NCT02556736. https://clinicaltrials.gov/study/NCT02556736?term=NCT02556736&rank=1.
22. Yan B, Viswanathan S, Brodie SE, et al. A clinically viable approach to restoring visual function using optogenetic gene therapy. Mol Ther Methods Clin Dev. 2023;29:406-417.
23. BS01 in Patients With Retinitis Pigmentosa. Updated May 3, 2023. National Clinical Trials Identifier NCT04278131. https://clinicaltrials.gov/study/NCT04278131?term=NCT04278131&rank=1.
24. Bionic Sight’s BS01 gene therapy receives RMAT designation from the FDA [press release]. New York, NY; BioSpace; February 18, 2025. Accessed March 4, 2025. https://www.biospace.com/press-releases/bionic-sights-bs01-gene-therapy-receives-rmat-designation-from-the-fda.